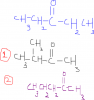http://onlineexamhelp.com/wp-content/uploads/2013/10/9701_s13_qp_22.pdf
Question 5 part d)i) how is it ethanoic acid?
http://onlineexamhelp.com/wp-content/uploads/2013/10/9701_s12_qp_22.pdf
Question3
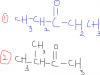
HO2CCH2CH(OH)CO2H <--That’s the acid given
and then in part B the reaction is heat with CH3CO2H/H+ <--This to is an acid...
so the Mark scheme says HO2CCH2CH(OH)CO2H (with CH3CO2H/H+)-->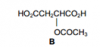
How did that form?
Question 5 part d)i) how is it ethanoic acid?
http://onlineexamhelp.com/wp-content/uploads/2013/10/9701_s12_qp_22.pdf
Question3
HO2CCH2CH(OH)CO2H <--That’s the acid given
and then in part B the reaction is heat with CH3CO2H/H+ <--This to is an acid...
so the Mark scheme says HO2CCH2CH(OH)CO2H (with CH3CO2H/H+)-->
How did that form?