- Messages
- 1,229
- Reaction score
- 740
- Points
- 123
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
High charge density increases covalent character. The cation would be able to attract the electron cloud of the anion, resulting in a slight sharing of the electrons. Recall that sharing of electrons basically means covalent bonding.View attachment 51123 can somebody explain this example
High charge density causes increase in covalent charater OR ionic character? :s
Start by grouping the elements into types of bondingWhat is the reason for the change in the shape of the graph for BOILING and Melting Points? :/
Are you confused between C and D?
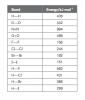
Do I need to study the H2 Singapore papers too, for chemistry? It seems significantly harder than the ones we have to do, so I thought it'll make good practice papers. But, does the questions there differ much from our syllabus? Or studying Intl' AS and A level papers would be sufficient?
when do we consider cis trans separately and when together?
why is ans C not D?
Consider all possibilities whenever applicable e.g. cis-cis, cis-trans, trans-cis, trans-trans
Post your diagrams and I'll try to tell you where you went wrong.
I agree, but the example says increase in ionic character :sHigh charge density increases covalent character. The cation would be able to attract the electron cloud of the anion, resulting in a slight sharing of the electrons. Recall that sharing of electrons basically means covalent bonding.
Start by grouping the elements into types of bonding
metallic (Na,Mg,Al) among metals, more delocalise electrons, higher mp/bp
giant covalent (Si) strongest among the 3 types of bonding.
simple covalent (P, S, Cl, Ar) , among simple molecules, higher Mr, higher mp/bp
Start by grouping the elements into types of bonding
metallic (Na,Mg,Al) among metals, more delocalise electrons, higher mp/bp
giant covalent (Si) strongest among the 3 types of bonding.
simple covalent (P, S, Cl, Ar) , among simple molecules, higher Mr, higher mp/bp
The book is incorrect.I agree, but the example says increase in ionic character :s
Right, but the picture i posted had separate graphs for M.P and B.P that were Not exactly same.. so my question is why not
even if those single bonded carbon atoms are double bonded with other carbon atoms?In this case, the cis-trans is the same as the trans-cis. So your 2nd and 4th are essentially the same molecules.
The often neglected part is the the molecule can rotate (twist) along the C-C single bond.
View attachment 51212
even if those single bonded carbon atoms are double bonded with other carbon atoms?
http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_s09_qp_1.pdf
mcq 5 and 6. I am stuck, can someone explain me the answers?
hey metonia, I am done with q6 but i was still wondering for question 5, why can't option B and D be the answers?Hi there, could you share your thoughts on the 2 questions so I know why you are stuck.
For almost 10 years, the site XtremePapers has been trying very hard to serve its users.
However, we are now struggling to cover its operational costs due to unforeseen circumstances. If we helped you in any way, kindly contribute and be the part of this effort. No act of kindness, no matter how small, is ever wasted.
Click here to Donate Now