- Messages
- 130
- Reaction score
- 81
- Points
- 28
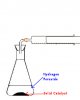
Man,u saved my life..Thnx alootChemistry | Test of ionsSo, on Wednesday we will have Chemistry paper 6. Every year there are test of ions based questions. Here is a list compiled by me on test of ions. Hope it helps!
Please tell me if there is any mistake in the list and I'll make changes A.S.A.P
best of luck