- Messages
- 187
- Reaction score
- 976
- Points
- 103
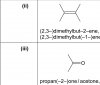
I don't think it will work because nitric acid is a strong acid which neutralized MgO, on the other hand KNO3 is almost neutral and is moderately soluble in water. I doubt if it'll react at all and of course to be on the safe side, we'd better write what we already came across in our syllabusView attachment 60328
Part (ii). I was wondering is it acceptable if the reagent is some nitrate salt of a metal more reactive than Mg like KNO3 or NaNO3 so a double displacement reaction takes place.