-
We need your support!
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Chemistry: Post your doubts here!
- Thread starter XPFMember
- Start date
- Messages
- 603
- Reaction score
- 1,102
- Points
- 153
Please check #9659 on page 583
- Messages
- 603
- Reaction score
- 1,102
- Points
- 153
Just uploaded
Nov 2013 P11
http://www.youtube.com/playlist?list=PL4Jmce8VJnNU4YaGfNrPYv7SBJXnB1IAC
June 2009 P1 (mistakenly typed as 2007 originally)
http://www.youtube.com/playlist?list=PL4Jmce8VJnNVom2AGZXz6fdxSnIl9G-8D
For those who messaged me to ask, I'ld like to clarify that I did not do these videos for my current students.
We actually are taking a different chemistry paper from 9701.
Nov 2013 P11
http://www.youtube.com/playlist?list=PL4Jmce8VJnNU4YaGfNrPYv7SBJXnB1IAC
June 2009 P1 (mistakenly typed as 2007 originally)
http://www.youtube.com/playlist?list=PL4Jmce8VJnNVom2AGZXz6fdxSnIl9G-8D
For those who messaged me to ask, I'ld like to clarify that I did not do these videos for my current students.
We actually are taking a different chemistry paper from 9701.
Last edited:
- Messages
- 70
- Reaction score
- 171
- Points
- 43
please help me out in:
http://papers.xtremepapers.com/CIE/... AS Level/Chemistry (9701)/9701_w11_qp_12.pdf
18 28 35 39
Thanks
http://papers.xtremepapers.com/CIE/... AS Level/Chemistry (9701)/9701_w11_qp_12.pdf
18 28 35 39
Thanks
- Messages
- 59
- Reaction score
- 130
- Points
- 43
Anyone please explain thesePlease help with these...i will really appreciate it!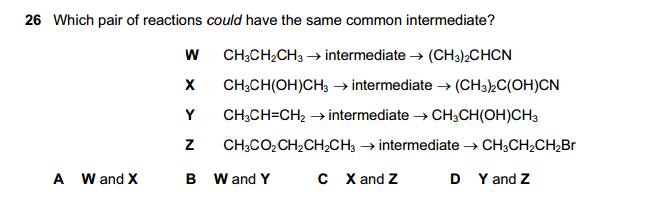
Ans B
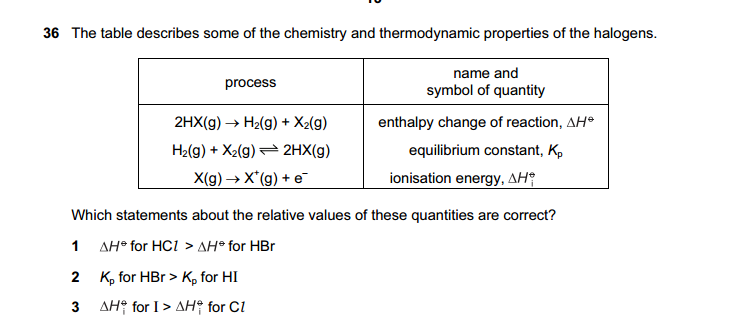
1 and 2 correct
the process is endothermic.It involved the breakage of the triple nitrogen bond...hey is the process endothermic??
- Messages
- 59
- Reaction score
- 130
- Points
- 43
http://papers.xtremepapers.com/CIE/... AS Level/Chemistry (9701)/9701_w12_qp_12.pdf
Number 11?? Anyone?? Answer is A how???
Number 11?? Anyone?? Answer is A how???
4)
P - One Br-Br bond is breaking. So enthalpy change is -193J
Q - One Cl-Cl bond is forming. So enthalpy change is +244J
R - One C-Cl bond is forming. So enthalpy change is +340J
S - One C-H bond is breaking. So enthalpy change is -410J.
Answer is therefore D.
6) NH4NO3 is really NH4+ and NO3-.
Oxidation state of N in NH4+:
x + 4 = +1
x = -3
Oxidation state of N in NO3- :
x + (-2 x 3) = -1
x = +5
Oxidation state of N in N2O:
2x + (-2) = 0
x = +1
So changes in oxidation numbers are:
-3 ---> +1 = +4
+5 ---> +1 = -4
Answer is D
21) Here, they're testing your knowledge of what happens in a free radical substitution reaction. A C-H bond is changed to a C-Cl.
In the compound given, work clockwise with the carbon atoms. In the top most carbon atom, any of the hydrogens being replaced will give the same X radical. So this gives one possible X radical. The next two carbons will also have only one possible distinct replacement. However, these replacements will give radicals which are identical to the replacement given by the first carbon. Try doing this on a paper if you don't get how.
Another possible replacement is on any one of the 2 hydrogens on the second-last carbon on the left. The last one is possible on any one of the 3 hydrogens on the last carbon.
Hence a total of 3 possible X radicals are possible. Ans: C
29) butan-2-ol can form only 2 straight chain alkenes plus one alkene with an alkyl side chain. Ans: B
40) in 3 both reactants are gases. They can't be 'heated under reflux'. Ans: B
I think you did a mistake with question 4.. R is -340 so ans is C
- Messages
- 12
- Reaction score
- 43
- Points
- 23
S11Q12
In this case, the cross multiplication does give us the answer, but have to be careful at A levels, not all species need to satisfy the octet rule.
E.g AlCl3 and SO4 2-
This was what i suggest some posts ago
Understand that whatever moles of C we have at the beginning will eventually be the same number of moles of C in CO2 at the end.
Let x and y be the ratio of Al and C respectively.
AlxCy --->???---> CO2
Working backwards from CO2,
moles of CO2 = 72/24 000 = 0.003 moles = moles of C in AlxCy
Going through the options
A Al 2C3, moles of Al2C3 = 0.144/90 = 0.0016, moles of C = 0.0016 x 3 = 0.0048 (incorrect)
B Al 3C4 , moles of Al3C4 = 0.144/129 = 0.0016, moles of C = 0.0016 x 4 = 0.0045 (incorrect)
C Al 4C3 , moles of Al4C3 = 0.144/144 = 0.001, moles of C = 0.001 x 3 = 0.003 (BINGO!)
D Al 5C3
Why did you multiply by 3? Here : " moles of C = 0.0016 x 3 = 0.0048 (incorrect) "
- Messages
- 5,330
- Reaction score
- 11,839
- Points
- 698
Q-A number of alcohols with the formula C4H10O are separately oxidised. Using 70 g of the alcohols
a 62 % yield of organic product is achieved.
What mass of product could be obtained?
1-42.2 g of butanone
2-51.6 g of butanoic acid
3-51.6 g of 2-methyl propanoic acid
working required please. especially Q6
thanks in advance
http://papers.xtremepapers.com/CIE/... AS Level/Chemistry (9701)/9701_s10_qp_12.pdf
hello can you please help me with these questions: 2,8,10,17,21,25,26,31 and 28
your help will greatly appreciated
hello can you please help me with these questions: 2,8,10,17,21,25,26,31 and 28
your help will greatly appreciated
- Messages
- 603
- Reaction score
- 1,102
- Points
- 153
Because 1 mole of Al2C3 has 3 moles of C.Why did you multiply by 3? Here : " moles of C = 0.0016 x 3 = 0.0048 (incorrect) "
So 0.0016 moles of Al2C3 has 0.0016 x 3 moles of C.
- Messages
- 603
- Reaction score
- 1,102
- Points
- 153
Please help with these...i will really appreciate it!
Ans B

1 and 2 correct
What year is this from? So I can label my replies for easier search next time.
Q26.
W: reacts with Br2 and uv to form intermediate CH3CHBrCH3 , which then reacts with CN-
X: reacts with Br2 and uv to form intermediate (CH3)2C(OH)Br ,which then reacts with CN-
Y: reacts with HBr to form intermediate CH3CHBrCH3 , which then reacts with aq. NaOH
Z: undergoes hydrolysis to form intermediate CH3CH2CH2OH , which then reacts with PBr3
Q39.
The first two statements requires being clear of about the concept that in terms of stability, HI is less stable, followed by HBr, then HCl.
Statement 1 (True)
It is harder for H-Cl to decompose to H2 and Cl2, compared to H-Br.
So for HCl , the reaction is more endothermic.
Statement 2 (True)
It is easier for HBr to form than HI.
So there is a larger ratio of products/reactants for HBr reaction than for HI.
So Kp for HBr is larger than HI
Statement 3. (False)
It is easier to remove an electron from I than Cl.
So IE for I is lower than Cl.
- Messages
- 603
- Reaction score
- 1,102
- Points
- 153
http://papers.xtremepapers.com/CIE/... AS Level/Chemistry (9701)/9701_w12_qp_12.pdf
Number 11?? Anyone?? Answer is A how???
w12qp12
Q11. This question does seem a bit confusing at the start. We rely on the definition of bond energy as much as possible to make our choices easier.
Bond energy is the energy required to break 1 mole of covalent bonds (with the species in gaseous phase)
Notice that option A is purely a bond breaking reaction, for the other options, there are bond breaking and bond formation, so its sensible to focus on option A.
However, option A, is actually breaking of n covalent bonds.
XYn --> nX + nY
So we need to take energy change of option A and divide by n, to get energy required to break 1 mole of covalent bonds
Its like
CH4 --> C + 4H
we need to divide this energy change by 4 to get the energy for 1 C-H bond.
Q23. It took me a while to understand what the question is describing.
The molecule must be broken in two and
1) contain same carbon atoms
2) both parts must have isomers
So this eliminates D, as 9 carbons molecule cannot form 2 smaller parts with equal carbons.
Isomers from when hydrocarbon have 4 or more carbon, so minimum we must have 8 carbons in the original chain to start with.
The bottom text is the examiners report, if what I typed earlier don't make sense.
"29% of candidates chose the correct answer, C. The most commonly chosen incorrect answer was A, chosen by 41% of candidates. If n=4 (option A) then the molecule C4H10 must be splitting to give C2H6 and C2H4, neither of which have structural isomers. The answer is C because if n=8 the products of cracking are
C4H10 and C4H8, both of which have structural isomers
Last edited:
- Messages
- 603
- Reaction score
- 1,102
- Points
- 153
View attachment 44876 View attachment 44877
Q-A number of alcohols with the formula C4H10O are separately oxidised. Using 70 g of the alcohols
a 62 % yield of organic product is achieved.
What mass of product could be obtained?
1-42.2 g of butanone
2-51.6 g of butanoic acid
3-51.6 g of 2-methyl propanoic acid
working required please. especially Q6
thanks in advance
Hi there, please include the answers next time so its easier for us to reply.
Q6. The heat of reaction in the equation is to break 6 N-F bonds, so to get the bond energy, we divide it by 6.
Q15. This is an often set question.
Group II nitrates decompose based on the equation below:
2 X(NO3)2 (s) --> 2 XO (s) + 4 NO2 (g) +O2(g)
Mass of XO = 5 -3.29 g = 1.52
Moles of XO = 1.52/ (Mr of X + 16)
Mass of X(NO3)2 = 5 g
Moles of X(NO3)2 = 5/(Mr of X + 124)
since moles of X(NO3)2 = moles of XO
5/(Mr of X + 124) = 1.52/ (Mr of X + 16)
We can solve for Mr of X using the equation , or you can substitute the Mr of the four options and do trial and error.
Last question
Mr of alcohol = 74
Moles of alcohol used = 70/74 = 0.946
Moles of alcohol converted into products = 0.946 x 62% = 0.586
1. If 0.586 mol of butanone was obtained, it would weight 0.586 x Mr of butanone = 0.586 x 72 = 42.2 g (statement 1 true)
2. If 0.586 mol of butanoic acid was obtained, it would weight 0.586 x Mr of butanoic acid = 0.586 x 88 = 51.6 g (statement 2 true)
3. If 0.586 mol of 2-methylpropanoic acid was obtained, it would weight 0.586 x 2-methylpropanoic acid = 0.586 x 88 = 51.6 g (statement 3 true)
- Messages
- 603
- Reaction score
- 1,102
- Points
- 153
please help me out in:
http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_w11_qp_12.pdf
18 28 35 39
Thanks
Please include the answers to make it easier for us to reply.
w11qp12
Q18.
Old lime mortar produces a gas when reacted with HCl, so it should be CaCO3 (to produce CO2) , instead of CaO and Ca(OH)2.
This leaves us with Options A and D.
A: CaO is harder than CaCO3
D: Ca(OH)2 is softer than CaCO3
So option D is more likely
Q28. cyclohexene is alkene, cyclohexanol is alcohol, so you have to choose the one reactant that reacts with one but not the other for distinguishing tests.
A. Is Tollen's reagent (no reaction with both)
B. Br2 (decoloursises in cyclohexene, remains brown in cyclohexanol)
C. 2,4 -DNPH (no reaction with both)
D. Reduction agent (no reaction with both)
Q35. During reaction where the mixture heats up, Ba(NO3)2 decomposes to BaO. Mg reacts with oxygen in air to form MgO.
Q39.
1. CH3CH2Cl --> CH3CH2OH
2. CH3CO2CH23 (ester undergo alkali hydrolyse) --> CH3CO2- + CH3OH --> CH3COOH + CH3OH
3. CH3CN --> CH3COO- --> CH3COOH
- Messages
- 603
- Reaction score
- 1,102
- Points
- 153
http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_s10_qp_12.pdf
hello can you please help me with these questions: 2,8,10,17,21,25,26,31 and 28
your help will greatly appreciated
Hi there, please include the answers next time to make it easier for us to reply.
s10qp12
Q2.
A. 1s2 2s2 2p1 ( 1 unpaired e in p orbital)
B. 1s2 2s2 2p6 3s2 3p1 ( 1 unpaired e in p orbital)
C. 1s2 2s2 2p6 3s2 3p3 (3 unpaired e in p orbital)
D. 1s2 2s2 2p6 3s2 3p6 3d1 4s2 (1 unpaired e in d orbital)
Q8.
X + Cl2 --> XCl2
moles of X = 2.92/Mr of X
moles of XCl2 = 5.287/ Mr of XCl2
since 1 mol of X produces 1 mol of XCl2
2.92/Mr of X = 5.287/ Mr of XCl2
2.92/Mr of X = 5.287/ (Mr of X + 71)
Either solve for Mr of X, or substitute Mr of Ba, Ca, Mg and Sr to see when Mr fits the equation.
Q10.
When temp increases, all particles have more energy, so both the forward AND the backward reaction speeds up.
For this particular reaction, although both directions speeds up, the backward reaction speeds up more than the forward reaction (eqm shifts left).
Q17. Both oxides are acidic.
P: 1s2 2s2 2p6 3s2 3p3 (no unpaired e in p orbital)
S: 1s2 2s2 2p6 3s2 3p4 (has 1 paired e in p orbital)
Q21. The first alcohol will not be dehydrated.
To dehydrate, we need to remove a OH from a carbon, and a H from a neighboring carbon.
The neighboring carbon in the 1st alcohol do have have a H that could be removed.
Q25.
This is a similar to question 22 of June 2013 paper 12, which i explained a video.
Q26.
rate of hydrolysis of primary halogen alkanes (sN2) is dependent on both the concentration of OH- and the halogen alkanes
rate of hydrolysis of tertiary halogen alkanes (sN2) is dependent only on the concentration of the halogen alkanes
since Y is not dependent of concentration of OH-, it is most likely a tertiary halogen alkane.
- Messages
- 59
- Reaction score
- 130
- Points
- 43
http://papers.xtremepapers.com/CIE/... AS Level/Chemistry (9701)/9701_w13_qp_13.pdf
Number 3 ?? Answer is B
Number 10?? Answer is A
Number 3 ?? Answer is B
Number 10?? Answer is A
- Messages
- 515
- Reaction score
- 1,447
- Points
- 153
Q 10:- ............ N2 + 3H2 -------------------> 2NH3http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_w13_qp_13.pdf
Number 3 ?? Answer is B
Number 10?? Answer is A
initial mole of N2 = 1
initial mole of H2 = 3
initial mole of NH3= 1.98
At equilibrium:-
mole of N2=> 1 - x = 1.64 so x= - 0.64
mole of 3H2=> 3 - 3x = 3-3(-0.64) = 3 + 1.92= 4.92
mole of 2NH3=> 1.98 + 2x = 1.98 + 2( - o.64) = 0.70
then the Kc value will be = (0.70)^2 / ((1.64) x (4.92)^3)
Note:- u have to take care of the -ve sign in the value of x