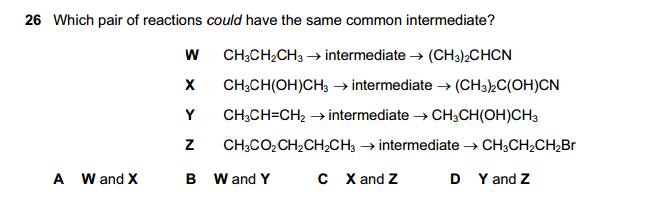
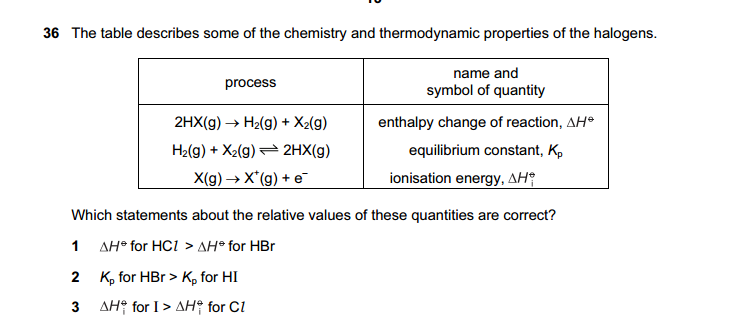
Which statements are true for an SN2 reaction?
1 One bond is broken as another bond is formed.
2 The formation of a transition state involves the collision of two molecules or ions.
3 A carbon atom in the transition state is bonded, either fully or partially, to five other atoms
1 One bond is broken as another bond is formed.
2 The formation of a transition state involves the collision of two molecules or ions.
3 A carbon atom in the transition state is bonded, either fully or partially, to five other atoms