- Messages
- 5
- Reaction score
- 18
- Points
- 13
hello, can anyone help me with multiple choice question 1,8 and 18 of 9701/01/M/J/04
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
Find the number of moles then multiply by the number of atom it contain, to get the moles of each atom.hello, can anyone help me with multiple choice question 1,8 and 18 of 9701/01/M/J/04
Hi guys
How are u all?
i need help in http://papers.xtremepapers.com/CIE/... AS Level/Chemistry (9701)/9701_w13_qp_12.pdf
Q8)
Q9)
Q13)
Q16)
Q17)
Q20)
THANK YOU GUYS
Even if u can help me only in few of them , I have NO PROBLEM
Thank you ...The "crossing of charges method" does give us the correct answer in this case, so if all else fails, I guess we can use it. However, lets see if we can make us of the other information given.
Understand that whatever moles of C we have at the beginning will eventually be the same number of moles of C in CO2 at the end.
Let x and y be the ratio of Al and C respectively.
AlxCy --->???---> CO2
Working backwards from CO2,
moles of CO2 = 72/24 000 = 0.003 moles = moles of C in AlxCy
Going through the options
A Al 2C3, moles of Al2C3 = 0.144/90 = 0.0016, moles of C = 0.0016 x 3 = 0.0048 (incorrect)
B Al 3C4 , moles of Al3C4 = 0.144/129 = 0.0016, moles of C = 0.0016 x 4 = 0.0045 (incorrect)
C Al 4C3 , moles of Al4C3 = 0.144/144 = 0.001, moles of C = 0.001 x 3 = 0.003 (BINGO!)
D Al 5C3
Hi guys
How are u all?
i need help in http://papers.xtremepapers.com/CIE/... AS Level/Chemistry (9701)/9701_w13_qp_12.pdf
Q8)
Q9)
Q13)
Q16)
Q17)
Q20)
THANK YOU GUYS
Even if u can help me only in few of them , I have NO PROBLEM

Thankyou so very much !!!Q1.
CxHy + O2 --> CO2 + H2O
from info, 10 cm3 of CxHy reacts with 50 cm3 of O2 to produce 30 cm3 of CO2
1CxHy + 5O2 --> 3CO2 + ?H2O
Comparing both sides of equation,
balancing carbon : x =3
balancing oxygen : 10 = 6 + ? , therefore ? = 4
1CxHy + 5O2 --> 3CO2 + 4H2O
Finally, balancing H: y = 8.
Q2.
From 1st reaction , 1 mole of NaN3 produces 1 mole of Na and 1.5 mole of N2.
The 1 mole of Na produced in 1st reaction will be used in the 2nd reaction, producing 0.1 mol of N2.
So total N2 produced = 1.5 (from 1st reaction) + 0.1 (from 2nd reaction) = 1.6 moles
Q13.
Be and Al has what we call a diagonal relationship.
Be2+ has a smaller charge than Al3+, but Be2+ has a smaller radius than Al3+. This causes their charge density (charge/radius) to be similar which in turns allows them to have similar chemical properties.
This also applies to Li and Mg and other pairings..
Google "diagonal relationship" for more details.
http://chemistry.tutorvista.com/inorganic-chemistry/s-block-elements.html
Q14. Unlikely that Ca's IE is higher than Mg, as the electrons of Ca are in a further shell.
Sum of Mg 1st 2 IE = 736 + 1450
Sum of Ca 1st 2 IE = 590 + 1150
Q38.
C2H4 + H2 --> C2H6
C2H4 --> polyethene (polymerisation)
C2H4 + cold KMno4 --> HOCHCHOH (forming diol)
june 2013Could I confirm the date of the chemistry paper?
A: initial: 2 moles of P
I solved this question by considering each option one at a time.http://papers.xtremepapers.com/CIE/... AS Level/Chemistry (9701)/9701_s12_qp_11.pdf
Number 9?? Answer B
Anyone please??
I solved this question by considering each option one at a time.
Option B)
.......2P <=> 2Q + R
I.......2...........0.......0
C....-2x........+2x....+x
E....2-2x........2x......x
2-2x + 2x + x
= 2 + x
Thnx manDo try to include the answers next to your selected questions, it would make it easier for us to reply.
Q8. To find the "heaviest atmosphere", we can use the weighted average of the Mr.
Using option D as example.
Weighted Mr of D
= 0.825 x Mr of H2 + 0.152 x Mr of He + 0.023 x Mr of CH4
=0.825 x 2+ 0.152 x 4 + 0.023 x 16
= 2.63
Work out the other 3 options, the highest one is the most dense.
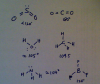
Q9.
View attachment 44395
Q13.
White precipitate means it forms an insoluble sulfate, which would be barium sulfate.
Q16.
Reaction 1: O.S of sulfur +6 (in H2SO4) --> +6 (in K2SO4), no change
Reaction 2: O.S of sulfur +6 --> +4 (in SO2) , change of 2 units
Reaction 3: O.S of sulfur +6 (in H2SO4) --> -2 (in H2S), change of 8 units
Q17. Ammonium chloride solution is slightly acidic as the NH4+ can donate protons (acidic), so it reacts with the alkaline magnesium hydroxide.
Q20.
1st reaction: oxidation (loss of H)
2nd reaction: nucleophilic addition (the lone pair on OH attracts the slightly positive carbon on the aldehyde)
3rd reaction : oxidation (loss of H)
http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_w08_qp_1.pdf
Help with 39 please! Answer is B, which is I & II.
w11qp12http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_w11_qp_12.pdf Q3 ans D ,Q26 ansA , Q31 ansA , Q35 AnsB , Q36 ansD Q37 ansD please help if you can
For almost 10 years, the site XtremePapers has been trying very hard to serve its users.
However, we are now struggling to cover its operational costs due to unforeseen circumstances. If we helped you in any way, kindly contribute and be the part of this effort. No act of kindness, no matter how small, is ever wasted.
Click here to Donate Now