- Messages
- 37
- Reaction score
- 28
- Points
- 18
Thank you, jazaakAllahu khairan. May Allah highly reward you for your help and for making others read your amazing quotes mashaAllah.Wa Alaykum Asalam Warahmatullahi Wabarakatuhu!
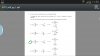
when i tried the question out i got B: 0.05 moldm-3 which i think you got too...but see here we have to read the question very carefully (this was a question already asked by someone and answered by another someone so that's how i understood)
it says "in the ratio of 15 g : 30 g : 15 g" NOT "in the ratio of 15 : 30 :15" Note: There IS a difference!
therefore:
Ans : A
- the percentage of N is the fertiliser is 15/100 = 0.15%
- in 14g of fertiliser, the amount of N is 14 x 0.15 = 2.1g
- the number of moles of N is 2.1g of N is 2.1/14 = 0.15 mole
- concentration = mole/volume, hence, 0.15/5 = 0.03moldm-3
(Jazak' Allahu Khairan to that person!)