- Messages
- 140
- Reaction score
- 414
- Points
- 73
No problem.Yes I got it. Thank you so much. I really appreciate it
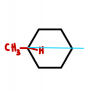
What if there were 6 C atoms in a ring, and one was bonded to CH3 and H? Is that C atom chiral?
Only a 6 Carbon ring with nothing else attached? f that is the case, then it wouldn't be a chiral centre.