- Messages
- 924
- Reaction score
- 1,096
- Points
- 153
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
Formation of ICl3 (s):
Oxidation number of N in:
Only B shows a p orbital.
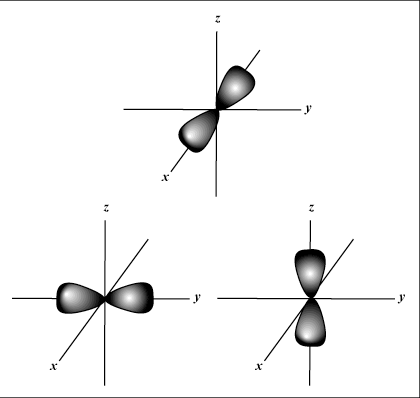
p orbitals
Formation of ICl3 (s):
1/2 I2 (s) + 3/2Cl2(g) --> ICl3(s)
2 x Formation of ICl3 (s) :
I2(s) + 3Cl2(g) --> 2ICl3(s)
How to do this?
Easy.
Atomize: I2(s) ---> I2(g) [+38]
React: I2(g) + 3Cl2(g) ---> 2ICl3 (s) [-214]
Total = 38 - 214 = -176kJ
But this was 2 x Formation of ICl3(s)
So divide it by 2. -176/2 = -88kJ/mol
Jazak Allah KhairanOxidation number of N in:
NH4+ : -3
NO3- : +5
N2O : +1
So change from NH4+ to N2O is +4
And change from NO3- to N2O is -4
Bring it onJazak Allah Khairan
You're a life saver!!!
I still have a few more doubts,If possible can you please solve them............
X is not a strong acid. The pH is only 6.
CaCO3 and Ca(OH)2 both react with acids. KNO3 is a salt. It is a result of neutralisation, not a reagent.
NO can be oxidised to NO2 in the atmosphere pretty easily. SO2 can also be oxidised to SO3, especially since NO catalyses this reaction.
Iodoform test I think A2 questions should be pretty much similar to the AS ones.So the 2016 syllabus for AS has somethings moved from A2 to As, namely carbon nanotubes and Buckminsterfullerene, infrared spectroscopy and iodofrom test. I have no where to practice questions from these so could anybody link some A2 papers having questions from these topics. Cheers
CaCO3 and Ca(OH)2 both react with acids. KNO3 is a salt. It is a result of neutralisation, not a reagent.
NO can be oxidised to NO2 in the atmosphere pretty easily. SO2 can also be oxidised to SO3, especially since NO catalyses this reaction.
Unfortunately, CO is quite stable and won't turn into CO2 in the atmosphere. If that was the case, we wouldn't be complaining about CO, since it would be naturally depleted. Only the CO2 produced would be an issue due to global warming.
Oxidation of NO and SO2 to form NO2 and SO3 respectively doesn't solve any problems, since it still forms acid rain and weathers the buildings and destructs aquatic life.
Those numbers indicate the chiral carbons right? But I am pretty sure number 9 is not a chiral carbon ... 1 to 8 are thoughhttp://www.chemguide.co.uk/basicorg/isomerism/cholesterol.gif
How do I identify the chiral carbon atom?
Ty in advance..
Those numbers indicate the chiral carbons right? But I am pretty sure number 9 is not a chiral carbon ... 1 to 8 are though
Yeah they do..but how am I supposed to know?
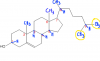
And the Carbons that are not numbered have either a double bond or have two Hydrogen atoms bonded to them. So they are fairly easy to eliminate from our list of chiral carbons.View attachment 59599
Consider a single Carbon atom. First, make sure that the Carbon has four bonds connected to it. If not, then draw the extra bonds and add the hydrogen.
Let's take Carbon 1 as our example.
Now, consider the groups attached to that Carbon. There is an - H, - OH. That much is obvious. Then look at the GROUPS attached on both sides. A common mistake is to just consider the Carbon ATOMS attached on either side and claiming the carbon to be a non-chiral centre. When you see the group on one either side, they are different (You know this because if you split the whole compound at that carbon, the two sides will not be symmetrical). So 4 different groups attached to Carbon 1. Therefore, Carbon 1 is a chiral centre.
If you repeat this with every other numbered Carbon, you will realise that Carbon 1 to Carbon 8 are chiral. However, Carbon 9 is not a chiral carbon because it has two CH3 groups bonded to it.
Hope you understand.
View attachment 59599
Consider a single Carbon atom. First, make sure that the Carbon has four bonds connected to it. If not, then draw the extra bonds and add the hydrogen.
Let's take Carbon 1 as our example.
Now, consider the groups attached to that Carbon. There is an - H, - OH. That much is obvious. Then look at the GROUPS attached on both sides. A common mistake is to just consider the Carbon ATOMS attached on either side and claiming the carbon to be a non-chiral centre. When you see the group on one either side, they are different (You know this because if you split the whole compound at that carbon, the two sides will not be symmetrical). So 4 different groups attached to Carbon 1. Therefore, Carbon 1 is a chiral centre.
If you repeat this with every other numbered Carbon, you will realise that Carbon 1 to Carbon 8 are chiral. However, Carbon 9 is not a chiral carbon because it has two CH3 groups bonded to it.
Hope you understand.
Konstantino Nikolas already gave an excellent answer. I just wanna add one point.Yeah they do..but how am I supposed to know?
For almost 10 years, the site XtremePapers has been trying very hard to serve its users.
However, we are now struggling to cover its operational costs due to unforeseen circumstances. If we helped you in any way, kindly contribute and be the part of this effort. No act of kindness, no matter how small, is ever wasted.
Click here to Donate Now