- Messages
- 603
- Reaction score
- 1,102
- Points
- 153
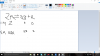
Plz also tell how to solve Q2 (c)(III) using the formula Ka= (H+)(salt) / (acid )
oct 06
Ka= (H+)(salt) / (acid )
log Ka = log [(H+)(salt) / (acid )]
log Ka = log (H+) + log [(salt)/(acid )]
-log (H) = - log Ka + log [(salt)/(acid )]
pH = pKa+ log [(salt)/(acid )]
pH = 7.2 + log (0.002/0.005)