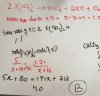- Messages
- 603
- Reaction score
- 1,102
- Points
- 153
You are referring to the line
mole of H2 formed = 33.3/100 *2 ?
The 2 is the total moles of gases, moles of H2 = % of H2 x total moles
mole of H2 formed = 33.3/100 *2 ?
The 2 is the total moles of gases, moles of H2 = % of H2 x total moles