- Messages
- 3,225
- Reaction score
- 15,036
- Points
- 523
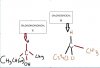
First tell me that can I right Co2 instead of Carboxylic Acid when Alkene undergoes reaction with KMNO4 hot concentrated. because like the end product of hot Kmno4 is always co2 gas
Secondly.. how B and C are chiral ? (written in Ms) like they dont have different functional group attached to it.

9701 paper 23 May june 2012.
Suchal Riaz
Secondly.. how B and C are chiral ? (written in Ms) like they dont have different functional group attached to it.

9701 paper 23 May june 2012.
Suchal Riaz