- Messages
- 405
- Reaction score
- 391
- Points
- 63
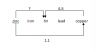
Is there a space given to write the answer? If yes then write it, if not then still write it. No harmthxxxxxxxxxxx, its correctbut if we just draw is thata full mark for the whole question? or do we need to write each Gr atom is bounded by 4 others?