- Messages
- 310
- Reaction score
- 125
- Points
- 53
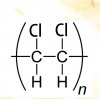
It says salt formation so the ratio of acid to calcium would be 2:1 for example for Butanedioic acid seperate into two parts and react it with calicum and it will look like this :View attachment 63791
I really can't figure this out. How can the formula be like that? Isn't it going to be 2 Ca ? The answer is C btw. Helppp
CH2COO- + Ca2+ = (CH2COO)2 Ca
And do that for the rest and so its formula will become
C8H8O8Ca2
Divide by 2 and so the E.F becomes
C4H4O4Ca