-
We need your support!
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Chemistry: Post your doubts here!
- Thread starter XPFMember
- Start date
- Messages
- 924
- Reaction score
- 1,096
- Points
- 153
I spot 7How do we figure out the number of chiral centres present??
Correct answer is 6.
- Messages
- 603
- Reaction score
- 1,102
- Points
- 153
How do we solve this?!!
Two glass vessels M and N are connected by a closed valve.
M contains helium at 20 °C at a pressure of 1 x 10^5 Pa. N has been evacuated, and has three times the volume of M. In an experiment, the valve is opened and the temperature of the whole apparatus is raised to 100 °C.
What is the final pressure in the system?
a. 3.18 x 10^4 Pa
b. 4.24 x 10^4 Pa
c.1.25 x 10^5 Pa
d.5.09 x 10^5 Pa
Here is what I did, Idont know if there is another way of solving it or not. Anyhow this is how i did it.
First I found the volume of M in terms of n using PV=nRT (there is no way of actually finding the number of moles because they didnt give you the mass of Helium or its volume) Anyhow
Volume of M --> 1x10^5*V=n*8.31*(20+273)
V=0.024n
Now they told you that the volume of N is three times that of M hence Vol of N is 3*V i.e 3*0.024n = 0.073n
Now they asked for the final pressure which means the pressure of both the gasses M and N and they told you that the temp is 100 so what we do is first we find the TOTAL volume of both M and N which is 0.024n+0.073n= 0.097n
so to find the Total pressure just put in your values in the formula
P*0.097n=n*8.31*(100+273) (the n will cancel out)
P=3.18x10^4
BTW when you attempt solving it yourself use the exact values from the calculator, if you use my rounded off values you will get 3.19x10^4
It is not necessary to find the number of moles (which are the same in the beginning and the end).
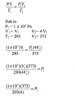
- Messages
- 603
- Reaction score
- 1,102
- Points
- 153
can any kind soul clear my doubts
here are the answers
1.C
7.C
10.A
12.D
14.B
25.C
Q1. Form a balance equation for the reaction.
Pb4+ + 2Br- --> Pb2+ + Br2
Moles of Pb4+ = 6.980/Mr of PbCl4
Work towards moles of Br2.....
Q7.
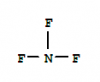
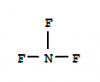
2 moles of NF3 are broken down into its atoms, so 6 moles of N-F bonds are broken.
Each N-F requires = + 1668/6 kJ of energy (positive as it is endothermic)
Q12.

Q14.
Group II nitrates decompose based on the equation below:
2 X(NO3)2 (s) --> 2 XO (s) + 4 NO2 (g) +O2(g)
Mass of XO = 5 -3.29 g = 1.52
Moles of XO = 1.52/ (Mr of X + 16)
Mass of X(NO3)2 = 5 g
Moles of X(NO3)2 = 5/(Mr of X + 124)
since moles of X(NO3)2 = moles of XO
5/(Mr of X + 124) = 1.52/ (Mr of X + 16)
We can solve for Mr of X using the equation , or you can substitute the Mr of the four options and do trial and error.
Q25.
75% of ethanol was converted = 0.75 x 2.76 = 2.07 g
ethanol --> ethanal
C2H5OH --> C2H4O
Moles of ethanol converted =?
Moles of ethanal formed =?
Mass of ethanal formed = ...
- Messages
- 1,229
- Reaction score
- 740
- Points
- 123
- Messages
- 117
- Reaction score
- 56
- Points
- 38
- Messages
- 117
- Reaction score
- 56
- Points
- 38
- Messages
- 117
- Reaction score
- 56
- Points
- 38
First of all the reaction is hemolytic fission. Then if we draw out the propogation and termination steps of reaction you can see if the chlorine atom joins at first or second carbon atom we get 1-chloropropane whereas we only get 2-chloropopane if it joins at second carbon atom. Hydrogen atoms at 2nd carbon are two and 1st and 3rd carbon are 6. the ratio will be 3:1why is it 1:3 & not 1:1
- Messages
- 2,206
- Reaction score
- 2,824
- Points
- 273
x = 104.5The answer is C but shouldn't it be A.
y = 109.5
z = 107.5
y -> z -> x
- Messages
- 117
- Reaction score
- 56
- Points
- 38
why is x 104.5 shouldnt it be 180x = 104.5
y = 109.5
z = 107.5
y -> z -> x
- Messages
- 2,206
- Reaction score
- 2,824
- Points
- 273
10)can any kind soul clear my doubts
here are the answers
1.C
7.C
10.A
12.D
14.B
25.C
A) HSO3 is a proton acceptor. Proton acceptor is a base.
B) SO2 is a reductant.
C) 2H+ donated proton to SO3(-2). Hence its base.
D) SO3(-2) is an oxidant.
- Messages
- 2,206
- Reaction score
- 2,824
- Points
- 273
There are two lone pairs. H-O-H isnt 180 coz of lone pair, CO2 is pure sp hybridization without lone pair, hence its linear.why is x 104.5 shouldnt it be 180
- Messages
- 2,206
- Reaction score
- 2,824
- Points
- 273
PLEASE HELPPPPPPPPPP
Correct answer is B.
Energy = 200 * 4.18 * 30 = 25080J
0.0326mol ---> 25080J
1mol ----> ?
25080/0.0326 = 769325.1534 = 769 kJ/mol (Combustion is exothermic reaxn) = -769kJ/mol
- Messages
- 1,229
- Reaction score
- 740
- Points
- 123
oxidant is the one that gets reduced itself right?10)
A) HSO3 is a proton acceptor. Proton acceptor is a base.
B) SO2 is a reductant.
C) 2H+ donated proton to SO3(-2). Hence its base.
D) SO3(-2) is an oxidant.
- Messages
- 1,229
- Reaction score
- 740
- Points
- 123
- Messages
- 2,206
- Reaction score
- 2,824
- Points
- 273
Use trial and error method.Omg, wth is this? -_-
Correct answer is D.
Take any random Hydrocarbon, let say C2H6 for instance (Try yourself with any hydrocarbon you want to).
A) C2H6 + 5O2 ---> 2CO2 + 3H2O (Not balanced).
B) C2H6 + 7/2O2 ---> 2CO2 + 6H2O (Not balanced).
C) C2H6 + 7/2O2 ---> 2CO2 + 3/2H2O (Not balanced).
D) C2H6 + 7/2O2 ---> 2CO2 + 3H2O (balanced).
- Messages
- 2,206
- Reaction score
- 2,824
- Points
- 273
Yes.oxidant is the one that gets reduced itself right?
- Messages
- 1,229
- Reaction score
- 740
- Points
- 123
- Messages
- 2,206
- Reaction score
- 2,824
- Points
- 273
Ethanenitrile and hydrochloric acid you would get ethanoic acid and ammonium chloride.Why is it A? :S ethane nitrile hydrolysis will make propanoic acid not ethanoic :s
CH3CN + 2H2O + HCl ---> CH3COOH + NH4Cl
Ref:
Acidic hydrolysis of nitriles
http://www.chemguide.co.uk/organicprops/nitriles/hydrolysis.html
- Messages
- 1,229
- Reaction score
- 740
- Points
- 123
http://maxpapers.com/wp-content/uploads/2012/11/9701_w14_qp_13.pdf
Q25
Ans is C but I got only 6 isomers. Can somebody tell me which ones am I missing?
Q25
Ans is C but I got only 6 isomers. Can somebody tell me which ones am I missing?





