- Messages
- 249
- Reaction score
- 419
- Points
- 73
oh okaayy thanks a lotyou could do that with H2 too.
number of moles of H2 used : number of moles of NH3 = 3:2
so (60 000 - 48 000):x=3:2
X=12 000*2/3=8000 moles = 136 Kg
http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_s12_qp_11.pdf
Q23 ?? :/ (ANS - B)
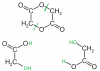
I don't really get the compound C drawn :S
can yu also help me with this, since no one is answerin :/