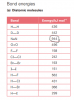
woopsthe answer is 1 ~
-
We need your support!
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Chemistry: Post your doubts here!
- Thread starter XPFMember
- Start date
- Messages
- 872
- Reaction score
- 894
- Points
- 103
Is carboxylic acid completely soluble in water?
YesIs carboxylic acid completely soluble in water?
- Messages
- 872
- Reaction score
- 894
- Points
- 103
Also isn't alcl3 tetrahedral and not trigonal planar??
yes please!I have learned it in another way and its a whole new concept but if you wanna know it i will explain my way it works everytime
- Messages
- 131
- Reaction score
- 220
- Points
- 43
http://papers.xtremepapers.com/CIE/...d AS Level/Chemistry (9701)/9701_w06_qp_1.pdf
Q3 (C), 4 (D), 21 (C), 25 (B)
Q3 (C), 4 (D), 21 (C), 25 (B)
It is trigonal planar as it Al has only 3 outer electrons as it is in group 3 and those three are covalently bonded to Cl so it has only 3 bond pairs and is trigonal planar.Also isn't alcl3 tetrahedral and not trigonal planar??
- Messages
- 872
- Reaction score
- 894
- Points
- 103
http://papers.xtremepapers.com/CIE/... AS Level/Chemistry (9701)/9701_s13_qp_11.pdf
Help in q30 please. Cant differentiate between B and D.
Help in q30 please. Cant differentiate between B and D.
2 is wrong as it is not high as it is formed from a reversible reaction and 3 is wrong as HSO4- is formed from a one way reaction but as the other is formed from a reversible one.
- Messages
- 872
- Reaction score
- 894
- Points
- 103
So HSO4 will be greater than SO4...but won't that shift the equilibrium to the right?2 is wrong as it is not high as it is formed from a reversible reaction and 3 is wrong as HSO4- is formed from a one way reaction but as the other is formed from a reversible one.
this is the new syllabus. At the time that question was made, 994 was used. Be my guest and try answering that question with 944.
- Messages
- 872
- Reaction score
- 894
- Points
- 103
In question. 11, one mole of the compound gives 3 moles of carbon so it's C since o.072/24 is .03 so the number if moles of the compound should be 1/3rd of the number of moles of carbon that it would produced-.01-so C is correctCan someone please look into these difficult questions?
View attachment 45028
View attachment 45029
View attachment 45030
- Messages
- 603
- Reaction score
- 1,102
- Points
- 153
How does solid iodine contain more then one type of bonding? Why is there only one kind in silicon dioxide?
Two kinds of bonding, covalent bonds between I and I atoms, intermolecular bonds among I2 molecules.
- Messages
- 872
- Reaction score
- 894
- Points
- 103
Is 23 B cause then it can follow a 1:2:1 ratio and ethens can form 50% of the productsCan someone please look into these difficult questions?
View attachment 45028
View attachment 45029
View attachment 45030
- Messages
- 297
- Reaction score
- 696
- Points
- 93
Guys i need ur help in this 
Q2 and 13 are done
Q2 and 13 are done
Metanoia , ZaqZainab , @ Every One, Could u Plz help me with this
http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_s05_qp_1.pdf
Q2..Answer B
Q13.Answer B>>>Why it is not Mg??Mg is just beside Al , so i thought that it will have a similar Electronegativity as Mg.
Q15.Answer C>>>When H2SO4 acts as an acid and when it acts as an oxidizing agent?
Q31.Answer A>>>I know that this is simple, but i am always confused with this type,So is there any hint??
Q32.Answer D
Q33.Answer B
Q40.Answer B
THNX in advance
- Messages
- 872
- Reaction score
- 894
- Points
- 103
Is 32 B. it behaves no ideally because volume is directly proportioned al to temperature for an ideal gas and it partially turns into a liquid because its volume decreases which means that the morgues are closing together and ate experiencing relatively stronger intermolecular forcesCan someone please look into these difficult questions?
View attachment 45028
View attachment 45029
View attachment 45030