-
We need your support!
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Chemistry: Post your doubts here!
- Thread starter XPFMember
- Start date
In 12 can't C be a option as well?Q11. The Cr is +6 before and after reaction.
Q12. Quite true, its was puzzling to me, the peak if shifted so far right would be really really low. So I prefer the other option. Its debatable.
Q18. That option is under transition metals, I believe is not required in your syllabus.
And in 18 if it is not in our syllabus how can we answer that question without knowing the structure?
And going back to question 9, after calculating bond energies the bond energy required to break D is actually smaller so how to answer that question.
And in question 20 why can't B be correct.
And in question 30 can you please draw me the structure as I don't understand the compound.
- Messages
- 603
- Reaction score
- 1,102
- Points
- 153
http://papers.xtremepapers.com/CIE/... AS Level/Chemistry (9701)/9701_w11_qp_12.pdf
Number 26 , Answer A
Anyone? please?
w11qp12
mass of ethanol converted to ethanal = 0.7 x 2.3 = 1.61 g
moles of ethanol converted to ethanal = 1.61/ 46 =0.035
moles of ethanal = 0.035 mol
mass of ethanal = 0.035 x 44 = 1.54 g
- Messages
- 201
- Reaction score
- 228
- Points
- 53
http://papers.xtremepapers.com/CIE/... AS Level/Chemistry (9701)/9701_w13_qp_12.pdf
Doubts : Q5 - C , Q22 -C , Q24 -A , Q25 -B , Q29 -D
THNX
Metanoia Browny
Doubts : Q5 - C , Q22 -C , Q24 -A , Q25 -B , Q29 -D
THNX
Metanoia Browny
- Messages
- 297
- Reaction score
- 696
- Points
- 93
am i invisible ???!!!! 
Guys could u plz help me with this
http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_s12_qp_11.pdf
DOUBTS:
Q13 --> ANSWER C
Q16 --> ANSWER C
Q19 --> ANSWER C
Q21 --> ANSWER C
- Messages
- 603
- Reaction score
- 1,102
- Points
- 153
In 12 can't C be a option as well?
And in 18 if it is not in our syllabus how can we answer that question without knowing the structure?
And going back to question 9, after calculating bond energies the bond energy required to break D is actually smaller so how to answer that question.
And in question 20 why can't B be correct.
And in question 30 can you please draw me the structure as I don't understand the compound.
Q12. Unfortunately, I feel its a poorly set question, as the scale is hard to compare. I feel peak of C is too high.
Q9. What I suggest is just a guide line, don't forget we need to factor in energy given out when H-O-H bonds are formed on the other side.
Q18. It is a paper that is set some years again, so its might be relevant then, but not this year.
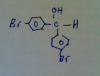
Q30. Look for the ester bond on the right. Cut the C-O, and reform the CH3CH2OCH3 on the right, into an alcohol.
Metanoia sorry but I needed the structure of A in 30.Q12. Unfortunately, I feel its a poorly set question, as the scale is hard to compare. I feel peak of C is too high.
Q9. What I suggest is just a guide line, don't forget we need to factor in energy given out when H-O-H bonds are formed on the other side.
Q18. It is a paper that is set some years again, so its might be relevant then, but not this year.
Q30. Look for the ester bond on the right. Cut the C-O, and reform the CH3CH2OCH3 on the right, into an alcohol.
And in 9 in all the reactions H2O are formed, right?
In 5 the rate should increase as the products which catalyse the reaction increase thereby elimination the answer D and also after some time as the reactants deacrease the reaction rate drops down as there are not enough reactants thereby answer being C.http://papers.xtremepapers.com/CIE/... AS Level/Chemistry (9701)/9701_w13_qp_12.pdf
Doubts : Q5 - C , Q22 -C , Q24 -A , Q25 -B , Q29 -D
THNX
Metanoia Browny
In 22 the answer is C as K2Cr2O7 is not strong enough to oxidise the double bond but is strong enough to completely oxidise the alcohol to a carboxylic acid as it is hot and concentrated.
In 29 all of them will react and form the required salt as some are esters and some are carboxylic acids.
For question 25 do you know how to upload a paint file to show you how to work it.
- Messages
- 116
- Reaction score
- 142
- Points
- 53
Hello! Can someone please explain how to solve Q 10
http://papers.xtremepapers.com/CIE/... AS Level/Chemistry (9701)/9701_w13_qp_13.pdf
http://papers.xtremepapers.com/CIE/... AS Level/Chemistry (9701)/9701_w13_qp_13.pdf
Metanoia I need structure of 30 A di(4-bromophenyl)methanol.
- Messages
- 603
- Reaction score
- 1,102
- Points
- 153
- Messages
- 297
- Reaction score
- 696
- Points
- 93
Guys could u plz help me with this 
http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_s12_qp_11.pdf
DOUBTS:
Q13 --> ANSWER C
Q16 --> ANSWER C
Q19 --> ANSWER C
Q21 --> ANSWER C
Browny
http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_s12_qp_11.pdf
DOUBTS:
Q13 --> ANSWER C
Q16 --> ANSWER C
Q19 --> ANSWER C
Q21 --> ANSWER C
Browny
- Messages
- 1
- Reaction score
- 3
- Points
- 1
Does anyone know what questions were given in summer 31 chemistry lab CIE???
- Messages
- 29
- Reaction score
- 91
- Points
- 13
Does anyone have any idea about what's coming in the practical 34 for chemistry tomorrow?
http://papers.xtremepapers.com/CIE/... AS Level/Chemistry (9701)/9701_s13_qp_13.pdf
can someone explain q8 19 22 28 35 37 39
can someone explain q8 19 22 28 35 37 39
- Messages
- 18
- Reaction score
- 40
- Points
- 23
Chem p3 tomorrow ,I need tips please  and any guesses whats coming
and any guesses whats coming