- Messages
- 603
- Reaction score
- 1,102
- Points
- 153
http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_w13_qp_11.pdf A
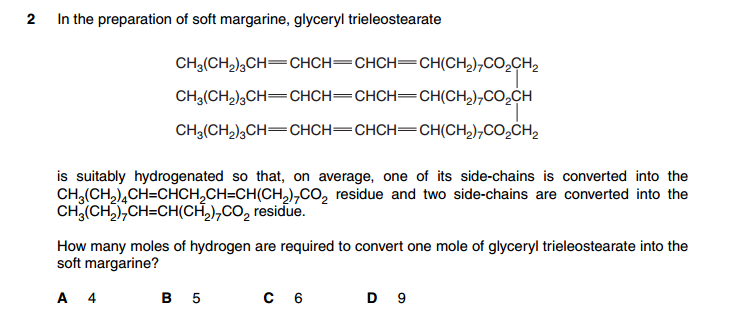
Number 26 Answer is B but why not 4??
w13qp11
4 atoms of H are removed (from 3 COOH and 1 OH).
4H --> 2H2 ( 2 moles of H2 gases)