- Messages
- 884
- Reaction score
- 449
- Points
- 73
Well the graph should not have shown 1.5 moles of oxygen thenWe take S8
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
Well the graph should not have shown 1.5 moles of oxygen thenWe take S8
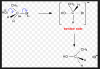
Attacks on CH3, CH3CH2 and CH2Help Needed urgent
thank youq18.
A) NH4+ does not NH3
B) NH3 is the one that accepts the H+
C) Changes from 107 in NH3 to 109.5 in NH4+, since NH4+ has a tetrahedral arrangement
D) This is the correct answer. H+ doesn't have any electrons, the bond is dative
Look at the mechanism to understand this better.
q4.
Only C has Hydrogen bonding which is stronger than the induce/permanent dipole IMFs of the other options.
q37.
Here's the mechanism:
View attachment 64036
Clearly 1 and 3 are correct, so the answer is A. I'm not sure what 2 is referencing exactly but I guess it's the collision between the OH ion and the haloalkane.
HEATED
Help and ExplainView attachment 64033 View attachment 64034
i have imaginary ceo friend of caies he says S8 is wrong...laahh
how B forms two different acids but D doesnt?q22.
Sketch the products when the hot Mno42-/H+ breaks the double bonds:
A) forms an acid and a ketone
B) forms two different acids
C) forms a dicarboxylic acid (two co2h gps attached to the same molecule)
D) forms two acids but they have the same formula
answer is B
how B forms two different acids but D doesnt?
thank you so much for your help
Electrolyisis is not in As 2018 syllabuswhat are electrodes made out of in electrolysis of brine?
I have never said S8 was wrong.What??i have imaginary ceo friend of caies he says S8 is wrong...laahh
Plz can u explain those 2 qs?
TysmI am sorry I just went offline minutes before you asked me but still I beleive I am not late
Q: 1 you asked is solved at the following website
http://chem-solutions-9701.blogspot.com/2016/11/chemistry-doubts-970111mj14.html
2 moles of a single product is formed just divide the formula of compound by 2
For question :2
For greatest amount of energy to be released the compound should not have oxygen in it or least amount for maximum energy released.
From examination report:
Question 9 Candidates were given four compounds – CH3CH2CH3 and three of its oxidation products, CH3CH2CH2OH, CH3COCH3 and CH3CH2CO2H – and asked which would release the greatest amount of energy on complete combustion. 40% of candidates gave the correct answer (propane itself), the others presumably not appreciating that every stage in the oxidation process would be exothermic, and that therefore the earliest point in a sequence of oxidations would yield the highest energy release. An alternative approach to this question would have been to make use of the table of bond energies within the Data Booklet, leading to the same conclusion. Rather surprisingly four questions had a facility below the design limit of 25%, i.e. below the ‘guessing level’, and the reasons for this were clearly very varied.
It is B...2nd is also correct since it is an anti oxidant
yes it does remove sour taste but I have schemes with this not as correct only anti oxidizing or reducing property and stop growth of aerobic bacteria are considered correct tohIt is B...2nd is also correct since it is an anti oxidant
For almost 10 years, the site XtremePapers has been trying very hard to serve its users.
However, we are now struggling to cover its operational costs due to unforeseen circumstances. If we helped you in any way, kindly contribute and be the part of this effort. No act of kindness, no matter how small, is ever wasted.
Click here to Donate Now