-
We need your support!
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Chemistry: Post your doubts here!
- Thread starter XPFMember
- Start date
- Messages
- 39
- Reaction score
- 29
- Points
- 28
]
its carbon environments so there are 3 different environments in the side chain and 4 different in the benzene ring itselfView attachment 63528
March 2017 Q6f(iii)
View attachment 63529
March 2017 Q7e(i)
Help me out any Chem Expert
anastasia grey113
- Messages
- 39
- Reaction score
- 29
- Points
- 28
- Messages
- 665
- Reaction score
- 13,609
- Points
- 503
is it Nitrogen and sulfur?i cant understand please help
for that u need to remember the test for sulfate ion
BaSO4 is insoluble unlike MgSO4
- Messages
- 39
- Reaction score
- 29
- Points
- 28
i got that part its the nitrogen and cl part how is overall charge of clo3 -1? m16 42 for your referenceis it Nitrogen and sulfur?
for that u need to remember the test for sulfate ion
BaSO4 is insoluble unlike MgSO4
- Messages
- 665
- Reaction score
- 13,609
- Points
- 503
well it could be cuz do u remember the react ion of chlorine with hot NaOH to make NaClO3?i got that part its the nitrogen and cl part how is overall charge of clo3 -1? m16 42 for your reference
in it Cl has a charge of +5.
that's how its -1.
- Messages
- 665
- Reaction score
- 13,609
- Points
- 503
but they said 'adjacent'groupsi got that part its the nitrogen and cl part how is overall charge of clo3 -1? m16 42 for your reference
then how come its Cl and N?
theyr not from adjacent groups
- Messages
- 665
- Reaction score
- 13,609
- Points
- 503
well i checked the mark scheme and it says sulfur and nitrogeni got that part its the nitrogen and cl part how is overall charge of clo3 -1? m16 42 for your reference
- Messages
- 3
- Reaction score
- 1
- Points
- 3
- Messages
- 39
- Reaction score
- 29
- Points
- 28
The state of br2 should be liquid i think and not aqHey guys, hope you guys are studying well for paper 4. I am wondering about this question, why when excess bromine is added, it only attaches to the phenyl group and doesn't add to the double bond by electrophilic addition. Is it a rule? Thanks and good luckView attachment 63538
Attachments
- Messages
- 665
- Reaction score
- 13,609
- Points
- 503
lol i dint quite get this eitherHey guys, hope you guys are studying well for paper 4. I am wondering about this question, why when excess bromine is added, it only attaches to the phenyl group and doesn't add to the double bond by electrophilic addition. Is it a rule? Thanks and good luckView attachment 63538
my teacher also dint get the concept behind this
but he said that if there are two reactions ans theyre asking for one, the one with phenol should be considered
- Messages
- 665
- Reaction score
- 13,609
- Points
- 503
lol idky its stated like this hereThe state of br2 should be liquid i think and not aq
but the bromine that is used is always dissolved in water
becuz if thats not done, bromine itself is a volatile liquid and would easily evaporate before all of the ethene reacts
- Messages
- 341
- Reaction score
- 224
- Points
- 53
https://papers.gceguide.com/A Levels/Chemistry (9701)/9701_s17_qp_42.pdf for Q3Biii why do we divide the answer in ii by 3? pls explain
- Messages
- 665
- Reaction score
- 13,609
- Points
- 503
Because in the previous part u calculated no. of moles of NaOH.https://papers.gceguide.com/A Levels/Chemistry (9701)/9701_s17_qp_42.pdf for Q3Biii why do we divide the answer in ii by 3? pls explain
Now consider both the equations
2NaOH + SO2----> Na2SO3 +H2O
NaOH + HCl ------> NaCl + H2O
so u see that three moles of NaOH r required to react with one mole of SO2 and 1 of HCl.
So to produce 1 mole of HCl and of SO2 the RCOOH should be 1/3 of the NaOH.
- Messages
- 341
- Reaction score
- 224
- Points
- 53
https://papers.gceguide.com/A Levels/Chemistry (9701)/9701_s17_qp_42.pdf for Q7 what is the diff b/w test 1 and 2? aren't the both to test for chloride ions?
- Messages
- 341
- Reaction score
- 224
- Points
- 53
can someone tell about the solubility of amides and amine in HCL? and with cold/hot hcl?
- Messages
- 438
- Reaction score
- 3,645
- Points
- 253
WHAT????????????????????????]
its carbon environments so there are 3 different environments in the side chain and 4 different in the benzene ring itself
- Messages
- 438
- Reaction score
- 3,645
- Points
- 253
too vaguelol i dint quite get this either
my teacher also dint get the concept behind this
but he said that if there are two reactions ans theyre asking for one, the one with phenol should be considered
- Messages
- 438
- Reaction score
- 3,645
- Points
- 253
- Messages
- 665
- Reaction score
- 13,609
- Points
- 503
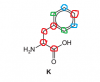
The ones I've circled in red are all having different chemcal environments...these are 5 carbons
The two in blue have the same env. as they are both next to the adjacent C atom in benzene next to the side chain so we will consider this 1 env.
Same goes for both in green...they are BOTH next to the Carbon side chain linked to benzene so both have same env. so v will consider this one env. too
so 1 + 1+ 5 = 7 different env.
Nickel will have cis trans isomers because it has two pairs of different kinds of ligands. Just draw two planar structures.
Also since Ni is not bonded to a bidentate or 4 different ligands, there should be no optical isomerism here.


