- Messages
- 122
- Reaction score
- 17
- Points
- 28
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
Moles of Iodine in the original solution= 0.2*50/1000=1x10-2how to do Question 1 ,part d (i)
When you plot you will get 2 patterns shown on the graph. One horizontal and the other slant line.Guys O/N 2015 P52 q2b part ii, please help. How do I draw the 2 best fit lines and MS even says something about horizontal line???
When you plot you will get 2 patterns shown on the graph. One horizontal and the other slant line.
Draw to different lines (one for the slant and one for the horizontal)
Extend both of the lines until they intersect, find the x value of the point of intersection.
I got 0.0248 (answers will obviously differ by a specific range)
Yes exactly last 3 points!I draw the horizontal line where the y value becomes same right? Like last 2-3 points?
But MS says 2 straight lines and then level off, do I need to draw 2 slanting "best fit" lines through the first few points excluding the horizontal points??? Or just simply 2 lines, one slanting and one horizontal??
Yes exactly last 3 points!
No no, just one best fit slant, and one horizontal (Last 3 points).... Like total of 2 best fits as the questions asks. And record the x value of the point of intersection.
Though 2 years aren't much but I did like from 2010, and the lastest ones are more important, so you'd do fine.Okay thank you so much. How's your prep for tmrw's p5? I'm writing paper 52, wbu? I've done papers only from 2014-2016.Any tips?
You got my inbox right? I am not allowed to post long passages, I don't even know why!Okay thank you so much. How's your prep for tmrw's p5? I'm writing paper 52, wbu? I've done papers only from 2014-2016.Any tips?
Draw a glass lid in between both the reactants, that can be dragged up for them to come in contact!Also any idea, how do we keep reactants separate until the reaction starts?
like maybe tie a thread to crucible and put the bung. then use thread to empty it. I think I read something like that
Though 2 years aren't much but I did like from 2010, and the lastest ones are more important, so you'd do fine.
Did you do all variants?
Also any idea, how do we keep reactants separate until the reaction starts?
like maybe tie a thread to crucible and put the bung. then use thread to empty it. I think I read something like that
I also read another method somewhere. That is to use a DIVIDED FLASK to separate the reagents. To start the reaction, just stir the flask, reactants will be mixed, and the reaction will start.Draw a glass lid in between both the reactants, that can be dragged up for them to come in contact!
OR
You can keep one reaction in some small cylinder which is attached to a small string. To start the reaction, the string is pulled, and the cylinder falls, causing the reactants to mix.
How do we draw thisI also read another method somewhere. That is to use a DIVIDED FLASK to separate the reagents. To start the reaction, just stir the flask, reactants will be mixed, and the reaction will start.
Divided flask is like this :How do we draw this
Plus the 2nd one I wrote was your method!!
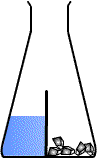
Oh its basically like glass lid, I mean this is how I usually draw it, without knowing that tis actually existDivided flask is like this :usually used to separate SOLID reactant from the LIQUID reactant. Used in those experiments for example: CaCO3 reacting with HCl etc

YesOh its basically like glass lid, I mean this is how I usually draw it, without knowing that tis actually exist
But yah thanks
Oww I see, thats nice!! I will label it as a divided flask right? And the examiner can later google itYesBut here you don't need to pull up that lid. That BARRIER is FIXED.
You just need to stir the flask to start the reaction. Pretty simple than pulling up the lid, or pulling the thread

Oww I see, thats nice!! I will label it as a divided flask right? And the examiner can later google it
For almost 10 years, the site XtremePapers has been trying very hard to serve its users.
However, we are now struggling to cover its operational costs due to unforeseen circumstances. If we helped you in any way, kindly contribute and be the part of this effort. No act of kindness, no matter how small, is ever wasted.
Click here to Donate Now