side chains decrease the contact between the molecules so vender waals forces become weaker and the material becomes weaker ..less dense and softerhttp://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_s12_qp_42.pdf
question 5 c(iv). Why can't we add a side chains ? Doesn't this increase van der waals force and so it becomes more rigid ?
-
We need your support!
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Chemistry: Post your doubts here!
- Thread starter XPFMember
- Start date
- Messages
- 46
- Reaction score
- 49
- Points
- 28
- Messages
- 924
- Reaction score
- 1,096
- Points
- 153
Well some endothermic reactions can occur readily. For example, salt dissolving is an endothermic process (sometimes) and it happens readily.
Thermal decomposition of CaCO3 at high temperatures "occurs readily".
Spontaneity of a reaction doesn't only depend on enthalpy change of it. Something known as entropy has to be taken into account as well. You'll learn more about entropy in A2
- Messages
- 340
- Reaction score
- 339
- Points
- 73
Even endothermic reacctions occur spontaneously. Actually, the spontaneity of reaction is determined by 2 factors: changes in enthalpy, Δ H and entropy. Though, mostly it's the Δ H that determines the spontaneity but even entropy matters, so since 3 says 'Only exothermic reactions can be made to occur readily', the use of word only makes it wrong, as endothermic can also be spontaneous
- Messages
- 340
- Reaction score
- 339
- Points
- 73
seems like we two wrote the same thing at the same time xDWell some endothermic reactions can occur readily. For example, salt dissolving is an endothermic process (sometimes) and it happens readily.
Thermal decomposition of CaCO3 at high temperatures "occurs readily".
Spontaneity of a reaction doesn't only depend on enthalpy change of it. Something known as entropy has to be taken into account as well. You'll learn more about entropy in A2
- Messages
- 924
- Reaction score
- 1,096
- Points
- 153
Haha yeahseems like we two wrote the same thing at the same time xD
- Messages
- 456
- Reaction score
- 280
- Points
- 73
- Messages
- 456
- Reaction score
- 280
- Points
- 73
- Messages
- 924
- Reaction score
- 1,096
- Points
- 153
When graphite is converted to diamond, energy is put IN to the system. It means diamond's bonds are weaker and longer than those of graphite. When you atomize both of them, you want to continue this process of weakening the bonds so they are completely separated. Since diamond already has slightly weaker bonds, lesser energy has to be put into it to achieve this.View attachment 59963 The Ans is A(all statements are correct). I get how statement 2 is correct but what about statement 1 and 3? How are they correct?:/
When you combust, you put energy into these species (the activation energy) and then ALL of it comes back out to the surrounding, and even more is released. (Exothermic reaction)
Therefore, since diamond has a little more energy put into it, it will release more energy. Or another way to think of it, less activation energy will be used on diamond, so the overall energy released will be greater.
In diagrams below, energy diagrams are shown. Diamond is in red. It shows the difference in energy changes in an endothermic reaction (atomization) and an exothermic reaction (combustion).
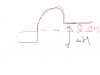

Last edited:
- Messages
- 27
- Reaction score
- 12
- Points
- 3
- Messages
- 924
- Reaction score
- 1,096
- Points
- 153
Should be 8.
There are 3 double bonds. Each can either be cis or trans.
So 2^3 = 8
- Messages
- 924
- Reaction score
- 1,096
- Points
- 153
Inititially, each of the three side chains has 3 double bonds.Ans is B, how? View attachment 59964
One is converted such that 2 double bonds are left. (i.e. One of it has undergone addition)
The other two are converted such that only 1 double bond is left. (i.e. Two double bonds have been removed)
So in total, the number of double bonds removed is 2x2+1 = 5.
Each double bond requires one mole of H2 to be hydrogenated, so your answer is 5.
- Messages
- 27
- Reaction score
- 12
- Points
- 3
YessShould be 8.
There are 3 double bonds. Each can either be cis or trans.
So 2^3 = 8
I had forgotten about that,
Thanks
can anyone help me with question 5(a) of O/N/09/42
http://papers.xtremepapers.com/CIE/... AS Level/Chemistry (9701)/9701_w09_qp_42.pdf
http://papers.xtremepapers.com/CIE/... AS Level/Chemistry (9701)/9701_w09_qp_42.pdf
- Messages
- 924
- Reaction score
- 1,096
- Points
- 153
I previously answered this here. See if it helps.can anyone help me with question 5(a) of O/N/09/42
http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_w09_qp_42.pdf
Thank you so much!!!!!I previously answered this here. See if it helps.
- Messages
- 27
- Reaction score
- 12
- Points
- 3
Can someone please post proper AS practical notes covering all main topics
Please!!!
Please!!!
http://papers.xtremepapers.com/CIE/... AS Level/Chemistry (9701)/9701_s12_qp_51.pdf
How can you prepare the different concentrations ?
- Messages
- 924
- Reaction score
- 1,096
- Points
- 153
Remember that last time you asked something very similar?View attachment 59985
http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_s12_qp_51.pdf
How can you prepare the different concentrations ?
It's the same steps I'm afraid
- Messages
- 924
- Reaction score
- 1,096
- Points
- 153
A Level Chemistry Paper 3 NotesCan someone please post proper AS practical notes covering all main topics
Please!!!
The above was created by Rizwan Javed so credit goes to him



