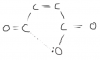- Messages
- 924
- Reaction score
- 1,096
- Points
- 153
If you're referring to this:http://studyguide.pk/Past Papers/CIE/International A And AS Level/9701 - Chemistry/9701_s08_qp_2.pdf
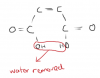
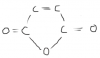
In this same paper part aii, why can't we write the structural formula like CO2H CO2H? why do we have to writeHO2CCO2H? I always get confused while writting structural formulas!SOMEONE?

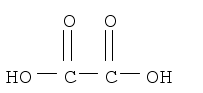
Then we must write this as:
HO2CCO2H
Or as:
HOOCCOOH
We cannot however, put:
CO2HCO2H,
Since the carbons are bonded together, and in this you cannot tell that the carbons are bonding, rather it seems as though the carbon is bonded to hydrogen!