-
We need your support!
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Chemistry: Post your doubts here!
- Thread starter XPFMember
- Start date
- Messages
- 603
- Reaction score
- 1,102
- Points
- 153
http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_w10_qp_11.pdf
question 29
idono why i cant solve isomer question everytime !!!! TT
An easy, albeit time consuming way to figure these questions out is to simply draw the isomers. You know there's a double bond, so it must show geometric isomerism - cis and trans, that makes 2. But they aren't asking for just geometric isomers, they're asking for all the isomers. Another one would be but-1-ene. So that makes 3. I know this isn't the best way to work it out, but it's better than nothing.
The question says 'structural or otherwise' :S I don't know, I could be wrong.
Could you or someone else help me with the questions I posted earlier? Just on the previous page.
but there is a differenc betweeen structural and stereo isomers isnt it :/?
well i think the cis and tra wont be included in this one :/
For this question, all isomers, structural, geometric (cis-trans) and optical (chiral) have to be considered.
Besides the 2-methylpropene the other 3 isomers are as mentioned by MaboroshI_I
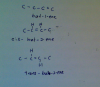
Its just that for C4H8, there is no chiral carbon, for we need no consider that part.[/quote]
who i n helll will think to do this correct only 1 mark omg jesus christ the merciful
- Messages
- 603
- Reaction score
- 1,102
- Points
- 153
MJ 2008 Q27!!!some1 plz explain
s08qp1
Q27.
Use the 4 options and trial and error.
Before dehydration, the C=C bonds were actually CH-COH.
Check which of the 4 could possibly give us a tertiary alcohol (resistant to oxidation) before dehydration.
View attachment 44341
In this case, it was D.
- Messages
- 603
- Reaction score
- 1,102
- Points
- 153
so whenester bond is broken only one hydroxyl group and one cooh group is formed right then na substitutes them to get alkoxide right
ester bond broken by acidic hydrolysis --> alcohol and COOH group
ester bond broken by alkaline hydrolysis --> alcohol and COO- group (carboxylate, not alkoxide)
5.175 then watGas X =N02
Gas Y =O2
2Mg(NO3)2 = 2MgO + 4N02 +O2
mass of gas X (NO2) produced =4*(mr of NO2)=4(46)=184
mass of gas Y (O2) produced =mr of O2=32
X/Y=184/32=5.75=1/0.174=5.75
answer = A
- Messages
- 872
- Reaction score
- 894
- Points
- 103
How do u convert C2H5OH into C2H5Cl?
- Messages
- 131
- Reaction score
- 220
- Points
- 43
mass of nitrogen in 14 g = 15/100 * 14 = 2.1help urGENT PLZZZZZZZZzView attachment 45080
moles of nitrogen = 2.1/14 = 0.15
conc in 5 dm3 = 0.15/5 = 0.03 mol dm-3
- Messages
- 131
- Reaction score
- 220
- Points
- 43
donno wht mistake am i committing
View attachment 45079
is it C? 940+2(-110)= +720
- Messages
- 46
- Reaction score
- 28
- Points
- 28
please,may i get helped in q# 35 for may june 2013 variant 13 , answer is C ?thanks
