You are asked to make dipetide out of 3 amino acids......so in how many ways can you arrange 2 from 3..i.e 3p2.......Thank you!
Just to make sure, could you justify why it is 3! for the first question and 3P2 for the second question? I mean, why not 3P3 for the second question? (since it also gives 6).
-
We need your support!
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Chemistry: Post your doubts here!
- Thread starter XPFMember
- Start date
- Messages
- 264
- Reaction score
- 395
- Points
- 73
I've done this question so I know what precedes the question. But, this is primarily why I dislike when sections of the question are cropped out and asked.
Look at the diagrams above the part you've posted. It has a benzene, a cyclohexane, a straight chain alkane (I think butane?) and a branched alkane. It's telling you that benzene and the straight alkane carbon atoms are coplaner while the branched + cyclic carbons are not. THIS concept is to be used here. You need to identify which of A, B, C, D and E have rings OR branching. If they do, they're not co-planer.
I believe apart from B, all others are co-planer.
C is coplaner because of the C-O-C linkage. O is on a different plane but since we need to consider C atoms, it's co-planer. All the C's are in the same plane in this. If you don't get why C is co-planer, ask me and I'll draw it out.
Why is Oxygen on a different plane? Sorry for asking so many (stupid) questions.
- Messages
- 675
- Reaction score
- 862
- Points
- 103
Why is Oxygen on a different plane? Sorry for asking so many (stupid) questions.
Ummm.. See the wedges
It has something to do with the lonepair + hybridization but I can't quite explain it.
See this.

The red part is the Oxygen.
- Messages
- 675
- Reaction score
- 862
- Points
- 103
1 b(vi)
Could you ttell me your answer to v? Would save me the trouble of going through all the steps.
Edit: Nevermind. Done them all anyway.
Original Solution: Fe3+ = 1.5 in 1000 so 0.15 in 100
Solution after dissolving copper several times: Fe2+ = Answer to part v/10 =0.6/10 = 0.06
Remaining Fe3+ = 0.15 - 0.06 = 0.09
Fe3+ : Cu
2 : 1
0.09 => 0.045mol
0.045 mol = 0.045*63.5 = 2.86g
Last edited:
[Fe2+] = 1.5 × 10–3 × 1000/2.5 = 0.6 (mol dm–3) ecf from (iv)Could you ttell me your answer to v? Would save me the trouble of going through all the steps.
Edit: Nevermind. Done them all anyway.
Original Solution: Fe3+ = 1.5 in 1000 so 0.15 in 100
Solution after dissolving copper several times: Fe2+ = Answer to part v/10 =0.6/10 = 0.06
Remaining Fe3+ = 0.15 - 0.06 = 0.09
Fe3+ : Cu
2 : 1
0.09 => 0.045mol
0.045 mol = 0.045*63.5 = 2.86g
Your ans is right
I got stuck myself at this stage :
"
Original Solution: Fe3+ = 1.5 in 1000 so 0.15 in 100
Solution after dissolving copper several times: Fe2+ = Answer to part v/10 =0.6/10 = 0.06
Remaining Fe3+ = 0.15 - 0.06 = 0.09 "
If you can elaborate this point if possible as how you did it .
- Messages
- 675
- Reaction score
- 862
- Points
- 103
[Fe2+] = 1.5 × 10–3 × 1000/2.5 = 0.6 (mol dm–3) ecf from (iv)
Your ans is right
I got stuck myself at this stage :
"
Original Solution: Fe3+ = 1.5 in 1000 so 0.15 in 100
Solution after dissolving copper several times: Fe2+ = Answer to part v/10 =0.6/10 = 0.06
Remaining Fe3+ = 0.15 - 0.06 = 0.09 "
If you can elaborate this point if possible as how you did it .
Original conc is 1.5 dm3
this means:
1.5 mols in 1000 cm3
so
0.15 mols in 100 cm3
Conc of Fe2+
0.6 mols in 1000 cm3
so
0.06 mols in 100cm3
- Messages
- 354
- Reaction score
- 529
- Points
- 103
Hey ! .. Can someone help me here !
http://papers.xtremepapers.com/CIE/...d AS Level/Chemistry (9701)/9701_w05_qp_1.pdf
Question 34,35,36
Thanks ^_^
http://papers.xtremepapers.com/CIE/...d AS Level/Chemistry (9701)/9701_w05_qp_1.pdf
Question 34,35,36
Thanks ^_^
- Messages
- 415
- Reaction score
- 252
- Points
- 53
Paper 5 , http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_w12_qp_53.pdf
Question 2b(iii) ( moles )
Question 3 , how to Complete the table > no formulas are given
& Whats is the max. Volume a gas syringe can hold which we are supposed to label here ?
AbbbbY
Question 2b(iii) ( moles )
Question 3 , how to Complete the table > no formulas are given
& Whats is the max. Volume a gas syringe can hold which we are supposed to label here ?
AbbbbY
Last edited:
- Messages
- 271
- Reaction score
- 429
- Points
- 73
Can someone plz help me with this chemistry question. 9701 october November 2011 paper 41 q6 bii.
Thanks!
Thanks!
- Messages
- 253
- Reaction score
- 1,195
- Points
- 153
Can someone plz help me with this chemistry question. 9701 october November 2011 paper 41 q6 bii.
Thanks!
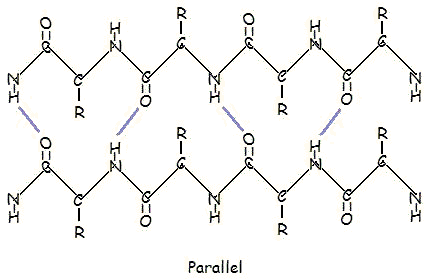
this is beta-pleated
you have to draw either an alpha-helix or beta-pleated and show the hydrogen bonding between them
- Messages
- 675
- Reaction score
- 862
- Points
- 103
Hey ! .. Can someone help me here !
http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_w05_qp_1.pdf
Question 34,35,36
Thanks ^_^
34:
I'd go with B. 3 is definitely wrong because calcium IONS wont react with H+, but solid will. 1 and 2 are correct. 2 is most definitely correct, and so I'm left with B.
35:
1- It does.
2- It does.
3- It doesn't because it's basic itself.
so, A
36:
B?
1- It is. NaClO3.
2- It does. +5 in NaClO3 and -1*5 in 5NaCl.
3- It doesn't. It's going from 6*+1 to +1 and 5*+1 so definitely not [R].
- Messages
- 675
- Reaction score
- 862
- Points
- 103

this is beta-pleated
Umm since they're beta pleated sheets, shouldn't they be running in opposite directions. I.e, CO under NH
- Messages
- 253
- Reaction score
- 1,195
- Points
- 153
they r not in diff directions.... in beta pleated hydrogen bonds form btw diff polypeptide chains and the r diff polypeptide chainsUmm since they're beta pleated sheets, shouldn't they be running in opposite directions. I.e, CO under NH
this diagram is lso given in my TB
Last edited:
- Messages
- 94
- Reaction score
- 29
- Points
- 28
http://papers.xtremepapers.com/CIE/... AS Level/Chemistry (9701)/9701_s13_qp_42.pdf
GUYS! Need help in paper 4!
Can someone explain question 1 Biv)
I have no clue how to calculate the rate, and dont understand the mark scheme. Pls thanks!
GUYS! Need help in paper 4!
Can someone explain question 1 Biv)
I have no clue how to calculate the rate, and dont understand the mark scheme. Pls thanks!
- Messages
- 40
- Reaction score
- 44
- Points
- 28
http://papers.xtremepapers.com/CIE/... AS Level/Chemistry (9701)/9701_s13_qp_42.pdf
Anyone generous to help
Q2 part b
Anyone generous to help
Q2 part b
- Messages
- 94
- Reaction score
- 29
- Points
- 28

Need help! why is the product at anode for MgBr is Br2? Isn't it suppose to be oxygen?
- Messages
- 271
- Reaction score
- 429
- Points
- 73
Thanks a lot!
this is beta-pleated
you have to draw either an alpha-helix or beta-pleated and show the hydrogen bonding between them
- Messages
- 94
- Reaction score
- 29
- Points
- 28

Guys! Need help! For the 3rd one, why is it no reaction? Isn't it carboxylic acid + alcohol reaction?
- Messages
- 271
- Reaction score
- 429
- Points
- 73
Umm since they're beta pleated sheets, shouldn't they be running in opposite directions. I.e, CO under NH
Can u plz show alpha helix also!
this is beta-pleated
you have to draw either an alpha-helix or beta-pleated and show the hydrogen bonding between them