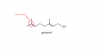
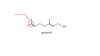
can u send me the link for that paper?
-
We need your support!
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Chemistry: Post your doubts here!
- Thread starter XPFMember
- Start date
- Messages
- 1,394
- Reaction score
- 1,377
- Points
- 173
c whether u make a curved line or a straight line u have to make it a best fit... i haven't solved the question but here's an example of the concept:
understood??
understood??
Where is the examplec whether u make a curved line or a straight line u have to make it a best fit... i haven't solved the question but here's an example of the concept:
understood??
- Messages
- 83
- Reaction score
- 114
- Points
- 43
O/N 2011 v.12help plzz
9701.mj.06 q, 25
9701.on.06 q,37,3
9701.mj.01 q 34,26,
9701.on.07 q 33,37,4
9701.mj.08 q 27 ,
9701.on.09 ( varient 11) 28,24,21
9701.on.10(varient 12) 39,27,13
9791.0n.10 (varient 11) q 38 ( what product will form if tollen reagent react with it ),35,29
9701.mj.10 (varient 11) q 12,
9701.mj.11(varient 12) q 38,29,28,24,11,
*9701.mj.11(varient 11) q 36,27,34,26,16
9701.on.11.(varient 12) q 9
9701.on.11 (varient 11) q 27,29,21,
9701.mj.12, (varient 12) q 24,23
9701.mj.12 (varient 11) q 29,23,22,9,
9701.on.12 (varient 11) q 26,
Q9. im sorry i cant explain this one
O/N 2011 v.11
Q21. Cl is easily separated from C compared to F so the molecule with more Cl and less F will cause most harm to the Ozone layer
Q29. answer is D check the pic for explanation
Q27. answer is D
it's easier for Cl to attach to outer carbon then inner carbon so there ration will be 3:1
and Cl always split by homolatic fission
M/J 2012 v.12
Q23 ans is B
C is not correct because the structure formed contain 2 Br atoms, and it is for when we react it with Br2 and not HBr!!
in D CHBr2CBr(CH3)CH3 should be inverse!
in A, the structure CH3CHBrCHBrCH3 is formed due to reaction with H2 gas and not HBr!
so our answer is B
Q24. from what i understood, in this one they are asking about the possible types of alkene an alkanes that can be made!
so the answer is all of them are possible thus it's A
Attachments
- Messages
- 140
- Reaction score
- 134
- Points
- 38
Thanks can u please tell me the equation for nitrile reduction1. Yes its in the AS topic ... Acid hydrolysis of nitriles produces carboxylic acid ...do learn its conditions and reagents.
2. Its an an topic ...u dont need to know about it
3 and 4. They falls under A2 organic.
* You should know about the reduction of nitriles (This equ is of both AS and A2..).
- Messages
- 140
- Reaction score
- 134
- Points
- 38
I just wanted to confirm that the varient for our practical is 33?
- Messages
- 24
- Reaction score
- 11
- Points
- 13
Asa,
I am really confuse in Q:1 of As Chemistry papers.
In that they ask questions like :
Element of the third period forms a chloride which is liquid at R.T.P?
An element which is liquid at R.T.P?
Let me know any source which i can read and solve these types of questions?
I am really confuse in Q:1 of As Chemistry papers.
In that they ask questions like :
Element of the third period forms a chloride which is liquid at R.T.P?
An element which is liquid at R.T.P?
Let me know any source which i can read and solve these types of questions?
- Messages
- 24
- Reaction score
- 11
- Points
- 13
Asa,
I am really confuse in Q:1 of As Chemistry papers.
In that they ask questions like :
Element of the third period forms a chloride which is liquid at R.T.P?
An element which is liquid at R.T.P?
Let me know any source which i can read and solve these types of questions?
I am really confuse in Q:1 of As Chemistry papers.
In that they ask questions like :
Element of the third period forms a chloride which is liquid at R.T.P?
An element which is liquid at R.T.P?
Let me know any source which i can read and solve these types of questions?
- Messages
- 140
- Reaction score
- 134
- Points
- 38
Asa,
I am really confuse in Q:1 of As Chemistry papers.
In that they ask questions like :
Element of the third period forms a chloride which is liquid at R.T.P?
An element which is liquid at R.T.P?
Let me know any source which i can read and solve these types of questions?
I think that the chloride of the element is liquid not the element for example, PCl3 is a volatile colourless liquid
- Messages
- 140
- Reaction score
- 134
- Points
- 38
Q2 bii
can someone plz explain how the first two structures given in the marking scheme are any different?
also, if i attach Cl to the carbon atom in the middle will it be accepted?
There r 2 structural isomers and one optical isomer of one of the structural isomers
- Messages
- 1,160
- Reaction score
- 3,858
- Points
- 273
Thanks can u please tell me the equation for nitrile reduction
CH3-CN + 4[H] ----------------> CH3-CH2-NH2
The H in the equation represents the Hydrogen from the R.A ...and R.A is (LiAl[H4] in dry ethaoxide ..or there's another condition for this reaction which is ..(the nitrile and hydrogen gas are passed over the nickle catalyst).
- Messages
- 1,160
- Reaction score
- 3,858
- Points
- 273
I just wanted to confirm that the varient for our practical is 33?
For this, you have to check your statement of entry, if you are appearing from zone 4 then the varients will be either 33 or 34.
- Messages
- 140
- Reaction score
- 134
- Points
- 38
CH3-CN + 4[H] ----------------> CH3-CH2-NH2
The H in the equation represents the Hydrogen from the R.A ...and R.A is (LiAl[H4] in dry ethaoxide ..or there's another condition for this reaction which is ..(the nitrile and hydrogen gas are passed over the nickle catalyst).
CH3-CN + 4[H] ----------------> CH3-CH2-NH2
The H in the equation represents the Hydrogen from the R.A ...and R.A is (LiAl[H4] in dry ethaoxide ..or there's another condition for this reaction which is ..(the nitrile and hydrogen gas are passed over the nickle catalyst).
Ok thanks
- Messages
- 222
- Reaction score
- 166
- Points
- 53
Hi guys have a question here.
http://papers.xtremepapers.com/CIE/... AS Level/Chemistry (9701)/9701_w10_qp_41.pdf
For the question 7c,i know part 1 cause it is memorising stuff.But for part 2 based on diagram,how do i know its octahedral.Is it memorising stuff too?
http://papers.xtremepapers.com/CIE/... AS Level/Chemistry (9701)/9701_w10_qp_41.pdf
For the question 7c,i know part 1 cause it is memorising stuff.But for part 2 based on diagram,how do i know its octahedral.Is it memorising stuff too?
- Messages
- 222
- Reaction score
- 166
- Points
- 53
- Messages
- 119
- Reaction score
- 181
- Points
- 53
http://papers.xtremepapers.com/CIE/...d AS Level/Chemistry (9701)/9701_w04_qp_1.pdf
q3 working?
q9, q23 and q28 how?
30, 37
38
39
q3 working?
q9, q23 and q28 how?
30, 37
38
39
- Messages
- 119
- Reaction score
- 181
- Points
- 53
http://papers.xtremepapers.com/CIE/...d AS Level/Chemistry (9701)/9701_w05_qp_1.pdf
q1 how can we find out moles needed? do we balance?
q1 how can we find out moles needed? do we balance?
Yeah we balance.http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_w05_qp_1.pdf
q1 how can we find out moles needed? do we balance?
Pb(C2H5)4 + 13.5O2 = PbO + 8CO2 + 10H2O
Therefore 1 mol requires 13.5 mol of O2.
http://papers.xtremepapers.com/CIE/...d AS Level/Chemistry (9701)/9701_w05_qp_1.pdf
Can anyone help me with questions 3,12,17?
Can anyone help me with questions 3,12,17?