- Messages
- 232
- Reaction score
- 103
- Points
- 38
AS is sooo easyI hate the fact we have to do AS organic as well
try A2 OMG every mark scheme has different answers, reactions and conditions :'(
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
AS is sooo easyI hate the fact we have to do AS organic as well
Not in a mood to identify more mistakesYou seem confident about yours answersshare them if you remember

TUTOR MEEEI find A2 organic comparatively easier
I find A2 organic comparatively easier
chem 52 ??? you are done with it ?
No, they were talking about Bio P52chem 52 ??? you are done with it ?
we're on the same page, bro.Agreed!
My chem paper 2 didnt go extremely well. Kinda disappointed. The paper just felt...different. InshaAllah aiming to make up for it in Paper 4!
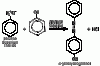
as you can see the second compound contains double bonds in the benzene ring while the first compound doesn't. Now you should know this that when Br2 is added to any alkene(double bond) it is decolourised. You should know this if you've done As level organiccan someone explain these observations ?
alright, see, the maximum electrons d shells and an s shells can hold are 10 and 2 respectively. If d shell contains 5 electrons which is half of the total number of electrons(10) it can hold then it is said to be stable.I would really appreciate if someone would help me with the configuration of chromium 3+ and manganese 2+ why are the electrons removed from the 4s orbital rather than 3d. Please help.
For almost 10 years, the site XtremePapers has been trying very hard to serve its users.
However, we are now struggling to cover its operational costs due to unforeseen circumstances. If we helped you in any way, kindly contribute and be the part of this effort. No act of kindness, no matter how small, is ever wasted.
Click here to Donate Now