- Messages
- 3,355
- Reaction score
- 20,175
- Points
- 523
Okay 24hrs have passed.... So here are the qstns i have compiled.... You can all discuss 
Btw how was phys paper 12/2015.... Expected gt?
Dont remember the sequence correctly: some of the answers mentioned are confirmed correct...
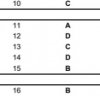
Q1 Which contains the same Number of electrons and neutron in 4 elements/ions/isotopes?(B)
Q2 An egg has mass of 50g. 5% is CaCO3. How much egg shells
required to neutralise 50cm^3 of 2.00mol/dm^3 of ethanoic acid?
Q3 Which compound or element has simple molecule lattice?(D)
Q4 PV=nRT. 103KPa of methane with volume of 5.37*10^-3(dont remember exact volume) at 60 degree temperature. Mass of methane?(D)
Q5 Purification of copper... What is correct order of anode cathode and change in solution?(A)
Q6 Which statement related to boltzman distribution curve is correct?(B)
Q7 Which statement related to silver ions and copper of glasses(shades) is correct?(A)
Q8 Which two area have reactants which form sodium chlorate? abestos diaphragm PQRS(P and S(Cl2 and NaOH))
Q9 Cholrate ions balancing?(C)
Q10 Which refractory material is X? X is heated to release CO2 and white solid. Y is used as refractory material.(C)
Q11 Which statement related to PH3 with H+ bonding is correct?(A)
Q12 Which statement related to Ammonium ion is correct?(D)
Q13 What were the temperature and pressure for N2O4 in equilibrium?(B)
Q14 Partial pressure Kp of HI?(A)
Q15 Ionization and electronegativity graph?(A)
Q16 What is the structure formed when an excess of HBr is used?(it was the one with both OH groups replaced with Br and the top of double bond had Br attached to it... Its either C or D)
Q17 Which will not make propene?(B)
Q18 Huge structure... When strong acid is used, which is the smallest structure?(B)
Q19 Which structure is optically active with six carbon atoms?(B, 2-methylpentan-3-ol)
Q20 Structure containing 3carboxylic acid and 1 OH. How much volume of hydrogen formed?(Dont remember this question exactly)(C)
Q21 3g of metallic nitrate is decomposed releasing 1.53g of gases. What is the structure?(D)
Q22 What is same in 31P and 37Cl?(D)
Q23 What are the interactions present Al2Cl6 which sublimes at idk what temperature?
Q24 What is true about exothermic reactions?
Q25 Which of the three are redox reactions?(B)
Q26 What is true about the lactic acid?(D)
Q27 What is correct for halogeno hydrocarbon?(A)
Q28 Which if these 3 structures lie in same plane?
Q29 What is formed when NaBH4 is used on CH3-C-HC(=CH2)(OH)-CH2-CHO(B)
Q30 What is the correct equation for the formation of Hexane structure?(the enthalpy qstn where correct equation was to be selected)(C)
Q31 Which statement of monopotassiumcitrate are correct?(dibasic, no chiral and it can react with NaHCO3)(A)
Q32 Dihalogenoalkane converted to Q and to dicarboxylic acid. What reagents are involved in Step 1 and 2?(D)
Q33 A mass of propan-2-ol was given to calculate the mass of propanone which yielded 74% mass.(A)
Q34 How may chiral carbons were present in the structure?(C, it was 7. Dont accurately which option waa 7)
If anyone of you remember anyother, plz tell me-i'll update my comment.
kitkat <3 :P Starlight97 Ahmed Aqdam asadalam KatnissEver Abdul Hanan hamzashariq
afrolina
Btw how was phys paper 12/2015.... Expected gt?
Dont remember the sequence correctly: some of the answers mentioned are confirmed correct...
Q1 Which contains the same Number of electrons and neutron in 4 elements/ions/isotopes?(B)
Q2 An egg has mass of 50g. 5% is CaCO3. How much egg shells
required to neutralise 50cm^3 of 2.00mol/dm^3 of ethanoic acid?
Q3 Which compound or element has simple molecule lattice?(D)
Q4 PV=nRT. 103KPa of methane with volume of 5.37*10^-3(dont remember exact volume) at 60 degree temperature. Mass of methane?(D)
Q5 Purification of copper... What is correct order of anode cathode and change in solution?(A)
Q6 Which statement related to boltzman distribution curve is correct?(B)
Q7 Which statement related to silver ions and copper of glasses(shades) is correct?(A)
Q8 Which two area have reactants which form sodium chlorate? abestos diaphragm PQRS(P and S(Cl2 and NaOH))
Q9 Cholrate ions balancing?(C)
Q10 Which refractory material is X? X is heated to release CO2 and white solid. Y is used as refractory material.(C)
Q11 Which statement related to PH3 with H+ bonding is correct?(A)
Q12 Which statement related to Ammonium ion is correct?(D)
Q13 What were the temperature and pressure for N2O4 in equilibrium?(B)
Q14 Partial pressure Kp of HI?(A)
Q15 Ionization and electronegativity graph?(A)
Q16 What is the structure formed when an excess of HBr is used?(it was the one with both OH groups replaced with Br and the top of double bond had Br attached to it... Its either C or D)
Q17 Which will not make propene?(B)
Q18 Huge structure... When strong acid is used, which is the smallest structure?(B)
Q19 Which structure is optically active with six carbon atoms?(B, 2-methylpentan-3-ol)
Q20 Structure containing 3carboxylic acid and 1 OH. How much volume of hydrogen formed?(Dont remember this question exactly)(C)
Q21 3g of metallic nitrate is decomposed releasing 1.53g of gases. What is the structure?(D)
Q22 What is same in 31P and 37Cl?(D)
Q23 What are the interactions present Al2Cl6 which sublimes at idk what temperature?
Q24 What is true about exothermic reactions?
Q25 Which of the three are redox reactions?(B)
Q26 What is true about the lactic acid?(D)
Q27 What is correct for halogeno hydrocarbon?(A)
Q28 Which if these 3 structures lie in same plane?
Q29 What is formed when NaBH4 is used on CH3-C-HC(=CH2)(OH)-CH2-CHO(B)
Q30 What is the correct equation for the formation of Hexane structure?(the enthalpy qstn where correct equation was to be selected)(C)
Q31 Which statement of monopotassiumcitrate are correct?(dibasic, no chiral and it can react with NaHCO3)(A)
Q32 Dihalogenoalkane converted to Q and to dicarboxylic acid. What reagents are involved in Step 1 and 2?(D)
Q33 A mass of propan-2-ol was given to calculate the mass of propanone which yielded 74% mass.(A)
Q34 How may chiral carbons were present in the structure?(C, it was 7. Dont accurately which option waa 7)
If anyone of you remember anyother, plz tell me-i'll update my comment.
kitkat <3 :P Starlight97 Ahmed Aqdam asadalam KatnissEver Abdul Hanan hamzashariq
afrolina
Last edited: