- Messages
- 64
- Reaction score
- 30
- Points
- 28
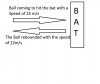
The change in velocity is calculated by the difference in final velocity and the initial velocity!
so for your example question it had be -100 km/h
i.e. Final - Initial = 400 - 500 = -100 km/h
Velocity can be affected by two things!
That is DIRECTION and THE SCALAR QUANTITY!
In your question both is changed!
Hope it helped!
so for your example question it had be -100 km/h
i.e. Final - Initial = 400 - 500 = -100 km/h
Velocity can be affected by two things!
That is DIRECTION and THE SCALAR QUANTITY!
In your question both is changed!
Hope it helped!