- Messages
- 155
- Reaction score
- 27
- Points
- 28
Question 1 (c) i!
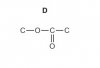
Another hydrocarbon, W, with the formula C4H8, reacts with hydrogen bromide, HBr, to give two products X and Y. X and Y are structural isomers of molecular formula C4H9Br. Reaction of X with aqueous alkali produces an alcohol, Z, that has no reaction with acidifi ed dichromate(VI).
It asks for the names of the structures. What I came up with was.
W = CH2=CHCH2CH3 - Butene
X= CH3CH2CH2CH2Br - Bromobutane
Y= CH3CH(Br) CH2CH3 - 2- Bromobutane
but in the mark scheme W,X and Y are different than my answers. I don't understand how my answers can be wrong. :/ Can someone PLEASE PLEASE explain why my formulas aren't considered correct since they have the same molecular formula as C4H8 and C4H9Br.
I'll be forever grateful!
I've attached the mark scheme!
http://maxpapers.com/wp-content/uploads/2012/11/9701_s14_ms_23.pdf
Another hydrocarbon, W, with the formula C4H8, reacts with hydrogen bromide, HBr, to give two products X and Y. X and Y are structural isomers of molecular formula C4H9Br. Reaction of X with aqueous alkali produces an alcohol, Z, that has no reaction with acidifi ed dichromate(VI).
It asks for the names of the structures. What I came up with was.
W = CH2=CHCH2CH3 - Butene
X= CH3CH2CH2CH2Br - Bromobutane
Y= CH3CH(Br) CH2CH3 - 2- Bromobutane
but in the mark scheme W,X and Y are different than my answers. I don't understand how my answers can be wrong. :/ Can someone PLEASE PLEASE explain why my formulas aren't considered correct since they have the same molecular formula as C4H8 and C4H9Br.
I'll be forever grateful!
I've attached the mark scheme!
http://maxpapers.com/wp-content/uploads/2012/11/9701_s14_ms_23.pdf