- Messages
- 59
- Reaction score
- 130
- Points
- 43
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
Chemistry P1 all hardhttp://papers.xtremepapers.com/CIE/... AS Level/Chemistry (9701)/9701_w10_qp_12.pdf
Number 7 and 8???? very hard . Anyone please explain them?
Any one know why the answer is B? I keep getting the ratio for the second part to be 1:1 not 1:2
Hahaha i know but can u do these 2 questions? if u can please explain them. ThanksChemistry P1 all hard:s
Q7 usehttp://papers.xtremepapers.com/CIE/... AS Level/Chemistry (9701)/9701_w10_qp_12.pdf
Number 7 and 8???? very hard . Anyone please explain them?
SRRY MANHahaha i know but can u do these 2 questions? if u can please explain them. Thanks
InshAllah!Thanks buddy
same to you
well honestly speaking its not that bad compared to how hard the Paper 1 is
i usually get around 30 now a days i used to get 20 before it all becaus eof practice
but then coming to the gtthey are too damn high for paper 1
you should score more than 30 to be on the safe side for paper 1
and as for paper 3 try just losing a mark or 2 or none cause paper 3 are very easy and they will safe you
InshAllah we all will get Good grades
as we want
In Q.2 i don't understand how only 5 double bonds are broken?
how come an ionic bond formed?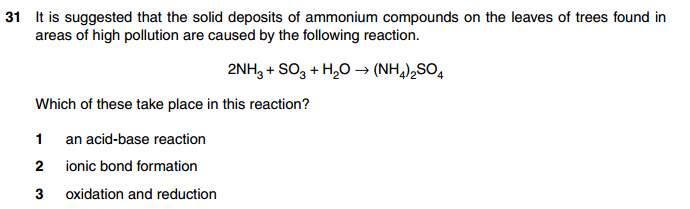
Both 1 and 2 are correct
http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_s12_qp_11.pdf Q19 ansC , Q22 ansA , Q27 ansD , Q36 ansA please help
http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_s07_qp_1.pdf
Q11
Q13can someone explain?
Q16 why not D? thats how HCl or HI is formed by breaking I-I bond and Cl-Cl bond
Q18
Q21
Q39 which two alkenes i have problems with ring structures
Q40
s04p1
Regarding Q28.
Key phrase in the question is "excess NaOH was used"
NaOH + SO3 --> NaHSO3 ---(1)
As there is excess NaOH, the HSO3- (acid) will further react.
NaHSO3+ NaOH --> Na2SO4 + H2O --(2)
Combine eqn (1) and (2), you will have the overall equation.
2NaOH + SO3 -->Na2SO4 + H2O
Q28. Tertiary alcohols can be oxidized by strong oxidizing agents (which is out of the syllabus), reason why they question use the phrase "not oxidized by mild oxidizing agents" is simply to reassure students that we are definitely talking about tertiary alcohols.
Q35 is as explained by ZaqZainab
Q19. Question is referring to NO2, are you asking about NO or NO2?
I could answer 5,6,25,30,33 if u figure out the rest plz tell me how to solve them as well!Can anyone PLEASE explain these question!
Q5,7,17,18,24,25,30,33,34 from
http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_w05_qp_1.pdf
http://papers.xtremepapers.com/CIE/Cambridge International A and AS Level/Chemistry (9701)/9701_w05_ms_1.pdf
For almost 10 years, the site XtremePapers has been trying very hard to serve its users.
However, we are now struggling to cover its operational costs due to unforeseen circumstances. If we helped you in any way, kindly contribute and be the part of this effort. No act of kindness, no matter how small, is ever wasted.
Click here to Donate Now