- Messages
- 129
- Reaction score
- 203
- Points
- 53
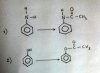
Well bro as far as my mind goes, it is D because they only said that 1 mole of the elements only. Not a certain mass (g). So According to the equation moles= Mass/Mr. If you find mass of the elements through this equation. You will find that we are burning more S than Al than Mg. So of-course burning an element in more mass requires more oxygen. Thus the answer is D.
If you have more doubts post them, i will try to help. And try to become more active in this thread specially
Hope this helps