Thank you so much for the reply. I wanted to ask why don't we multiply enthalpy change for sulfurtrioxide with 2 .First of all reverse the first reaction and multiply it by 2 as we do in writing equations for contact process this will also change the sign of enthalpy change required for this reaction i.e 2(296.5)=+593 make an overall equation of the both equations given to get 2SO2(g) + O2(g) → 2SO3(g)
then
-791.4+593= -198.4 thus D is correct option.
-
We need your support!
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Chemistry: Post your doubts here!
- Thread starter XPFMember
- Start date
- Messages
- 665
- Reaction score
- 13,609
- Points
- 503
cuz the reaction is like 2SO2 + O" -> 2SO3Thank you so much for the reply. I wanted to ask why don't we multiply enthalpy change for sulfurtrioxide with 2 .
so that means the enthalpy change already shows 2 moles being made n in oxidation the same no. of moles are made.
But in SO2 formation, only one mole is made in the reaction.
But in oxidation of SO2, 2 moles are used. So to equal the moles, it was multiplied.
a big big big thanks to you from my side. but isn't it a bit misleading to write -781.4 per mole. They could have just written enthalpy change = 781.4 . i hope i am not annoying you. this per mole thing is what is causing me the whole confusion anastasia grey113
- Messages
- 665
- Reaction score
- 13,609
- Points
- 503
its okaya big big big thanks to you from my side. but isn't it a bit misleading to write -781.4 per mole. They could have just written enthalpy change = 781.4 . i hope i am not annoying you. this per mole thing is what is causing me the whole confusion anastasia grey113
okay let me explain
We have one goal and that is MAKING 2 MOLES OF SO3.
let's start from the oxidation reaction
to make 2 moles of SO3 you need 2 moles of SO2 right?
So let's say we are given 1 mole of O2 and one mole of S and we make one mole of SO2 and we get an energy change of -791.4
Now we are asked to make 2 moles of SO3 from the product we got.
But we only have 1 mole of SO2 when we actually need 2 so do you think it's possible for the reaction to occur with just 1 mole of SO2?
According to the equation we need 2 moles of it.
So we will need to do the reaction twice to have two moles of SO2 for oxidation.
So we do the reaction twice. And the enthalpy becomes -791.4 x 2 right?
But for the formation of SO3 (i'm talking abt formation) 2 moles are already being made.
Now to answer your question.
PER MOLE MEANS the enthalpy change when one mole of the product is made.
Let's suppose we have 2 moles of S and two moles of O2, then obvio 2 moles of SO2 will be formed and the enthalpy change will be different right?
Even though the reaction is same.
So to clarify, they wrote per mole.
- Messages
- 150
- Reaction score
- 222
- Points
- 53
Hey Guys! Long time no see. I hope your studies are going great this year!
Could an A level student help me out here. Is there any easy way to memorize all the Transition metal complex reactions regarding Cobalt, Manganese, copper etc., and their products?
Could an A level student help me out here. Is there any easy way to memorize all the Transition metal complex reactions regarding Cobalt, Manganese, copper etc., and their products?
- Messages
- 665
- Reaction score
- 13,609
- Points
- 503
You don't need to revise all of them.Just go with cobalt and copper.Hey Guys! Long time no see. I hope your studies are going great this year!
Could an A level student help me out here. Is there any easy way to memorize all the Transition metal complex reactions regarding Cobalt, Manganese, copper etc., and their products?
- Messages
- 150
- Reaction score
- 222
- Points
- 53
Thanks, but is there any easy way to memorize the products and the reactions for cobalt and Copper ? Im having a really hard time with Ligands this year!You don't need to revise all of them.Just go with cobalt and copper.
- Messages
- 665
- Reaction score
- 13,609
- Points
- 503
Not really. You just have to memorise them as they are. There's no concept.Thanks, but is there any easy way to memorize
I hope you remember the colours of Cu on adding NH3.
They will help you memorise the colours of two reactions and that's that.
Otherwise it's all memorisation.
- Messages
- 438
- Reaction score
- 3,645
- Points
- 253
Hey where have you been all the time. Seeing you almost after a year though Welcome back to the XPC!Hey Guys! Long time no see. I hope your studies are going great this year!
Could an A level student help me out here. Is there any easy way to memorize all the Transition metal complex reactions regarding Cobalt, Manganese, copper etc., and their products?
Yes unfortunately you will have to memorize these reactions.
- Messages
- 46
- Reaction score
- 28
- Points
- 28
May june 2016/12, q#7 why the answer is D instead of C, any comments please?
- Messages
- 500
- Reaction score
- 419
- Points
- 73
May june 2016/12, q#7 why the answer is D instead of C, any comments please?
Are you sure you are substituting the values correctly.
T is an alcohol, CxHyO. A gaseous sample of T occupied a volume of 20cm3 at 120°C and 100kPa. The sample was completely burned in 200cm3 of oxygen (an excess). The final volume, measured under the same conditions as the gaseous sample, was 250cm3 . Under these conditions, all water present is vaporised. Removal of the water vapour from the gaseous mixture decreased the volume to 170cm3 . Treating the remaining gaseous mixture with concentrated alkali, to absorb carbon dioxide, decreased the volume to 110cm3 . The equation for the complete combustion of T can be represented as shown. CxHyO + zO2 xCO2 + y/ 2 H2O (i) Use the data given to calculate the value of x ..
the answer states that x= 3
How?????!!!!!!!!
Thx for help
the answer states that x= 3
How?????!!!!!!!!
Thx for help
- Messages
- 665
- Reaction score
- 13,609
- Points
- 503
Well first write down the volumes of each gas.T is an alcohol, CxHyO. A gaseous sample of T occupied a volume of 20cm3 at 120°C and 100kPa. The sample was completely burned in 200cm3 of oxygen (an excess). The final volume, measured under the same conditions as the gaseous sample, was 250cm3 . Under these conditions, all water present is vaporised. Removal of the water vapour from the gaseous mixture decreased the volume to 170cm3 . Treating the remaining gaseous mixture with concentrated alkali, to absorb carbon dioxide, decreased the volume to 110cm3 . The equation for the complete combustion of T can be represented as shown. CxHyO + zO2 xCO2 + y/ 2 H2O (i) Use the data given to calculate the value of x ..
the answer states that x= 3
How?????!!!!!!!!
Thx for help
1- The initial mixture with H2O + CO2 + O2 has a total volume of 250cm3 but removing H2O gave a volume of 170cm3.
So VOLUME OF H2O = 250cm3 - 170cm3 = 80cm3.
2- The remaining mixture of CO2 + O2 = 170cm3. Removing CO2 with alkali gives 110cm3.
So VOLUME OF CO2 = 170cm3 - 110cm3 = 60cm3.
3- The final mixture only contains O2 which is 110cm3. So out of 2o0cm3, 110cm3 did not react.
so VOLUME of reacted O2 = 200cm3 - 110cm3 = 90cm3.
4- Volume of alcohol = 20cm3.
Now since all are gases (remember water is actually water vapour so its a gas too) , we can take their ratios to find no. of moles.
so
CxHyO + zO2 -> xCO2 + y/2H2O
20 : 90 : 60 : 80
Now in order to find the value of x, we are supposed to take only 1 mole of the alcohol. So we will reduce the ratios down in such a way that the no. of moles of alcohol are 1.
So it'll be like
CxHyO : O2 : CO2 : H2O
1 : 4.5 : 3 : 4
The no. of moles of CO2 r 3 so x = 3.
- Messages
- 6
- Reaction score
- 3
- Points
- 13
Hi. I just want to ask.
In the data booklet there's so many standard electrode potential equation specifically for Mn04-. So which one should I use?
In the data booklet there's so many standard electrode potential equation specifically for Mn04-. So which one should I use?
- Messages
- 665
- Reaction score
- 13,609
- Points
- 503
When the question is related to reduction of acidified KMnO4, useHi. I just want to ask.
In the data booklet there's so many standard electrode potential equation specifically for Mn04-. So which one should I use?
MnO4 – + 8H+ + 5e ---> Mn2+ + 4H2O
- Messages
- 8,477
- Reaction score
- 34,837
- Points
- 698
Hi. I just want to ask.
In the data booklet there's so many standard electrode potential equation specifically for Mn04-. So which one should I use?
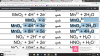
These are the three electrode potentials given to us in data booklet for MnO4-.
You can see on RHS of each equation, there are different species mentioned, namely MnO4(-2), MnO2 and Mn(+2), so now depending on to the data given to you in the question, you use respective electrode potentials.. I hope that made some sense.
Well first write down the volumes of each gas.
1- The initial mixture with H2O + CO2 + O2 has a total volume of 250cm3 but removing H2O gave a volume of 170cm3.
So VOLUME OF H2O = 250cm3 - 170cm3 = 80cm3.
2- The remaining mixture of CO2 + O2 = 170cm3. Removing CO2 with alkali gives 110cm3.
So VOLUME OF CO2 = 170cm3 - 110cm3 = 60cm3.
3- The final mixture only contains O2 which is 110cm3. So out of 2o0cm3, 110cm3 did not react.
so VOLUME of reacted O2 = 200cm3 - 110cm3 = 90cm3.
4- Volume of alcohol = 20cm3.
Now since all are gases (remember water is actually water vapour so its a gas too) , we can take their ratios to find no. of moles.
so
CxHyO + zO2 -> xCO2 + y/2H2O
20 : 90 : 60 : 80
Now in order to find the value of x, we are supposed to take only 1 mole of the alcohol. So we will reduce the ratios down in such a way that the no. of moles of alcohol are 1.
So it'll be like
CxHyO : O2 : CO2 : H2O
1 : 4.5 : 3 : 4
The no. of moles of CO2 r 3 so x = 3.
thanks a looot
- Messages
- 2
- Reaction score
- 0
- Points
- 1
Which process could be used to calculate the bond energy for the covalent bond X-Y by dividing its ΔH by n?
A. XYn (g) ---> X (g) +nY (g)
B. 2XYn (g) ---> 2XYn-1 (g) +Y2 (g)
C. Y (g) + XYn-1 (g) --->XYn (g)
D. nXY (g) ---> nX (g) + n/2Y2 (g)
A. XYn (g) ---> X (g) +nY (g)
B. 2XYn (g) ---> 2XYn-1 (g) +Y2 (g)
C. Y (g) + XYn-1 (g) --->XYn (g)
D. nXY (g) ---> nX (g) + n/2Y2 (g)
- Messages
- 500
- Reaction score
- 419
- Points
- 73
Which process could be used to calculate the bond energy for the covalent bond X-Y by dividing its ΔH by n?
A. XYn (g) ---> X (g) +nY (g)
B. 2XYn (g) ---> 2XYn-1 (g) +Y2 (g)
C. Y (g) + XYn-1 (g) --->XYn (g)
D. nXY (g) ---> nX (g) + n/2Y2 (g)
Consider a similar reaction e.g CH4------> C + 4H
For case of option A when we have to find the average bond energy of C-H we will divide ΔH by 4 which in this case we will divide it by n. (thus option A is correct)
In case of second and third option X-Y have not been completely broken down thus we cannot divide it's ΔH directly by n.
For the Last option there is a formation of Y2 molecules which means bond formation occurred.
In case of ΔH of bond energy only bonds are broken (bond breaking) occurs and NO bonds are formed. In bond breaking atoms are formed not molecules.
- Messages
- 438
- Reaction score
- 3,645
- Points
- 253
