- Messages
- 665
- Reaction score
- 13,609
- Points
- 503
okay first see what youve been provided withcan someone help PLEASE? :/
the volume, pressure, and temperature
what can you use here? pV=nRT
this will give you the number of moles of CxHy
rearrange the equation to make n=pV/RT
put in the values
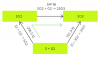
n = 100x103 x 25x10-6/8.31 x 310
the temperature is converted to Kelvins by adding 273 to 37
and volume is converted to cubic metres
the answer is 9.7x10-4
now its been told that when CO2 is absorbed, the volume changes from 150cm3 to 50cm3.
so to find out how much CO2 was produced, we subtract final volume from the initial one which gives us
V= 150 - 50 = 100cm3 <----- volume of CO2
now we have the volume of the gas and we know that one mole of any gas occupies 24dm3 or 24000cm3 of volume at rtp.
So we can use No. of moles = Volume/24000 to find the no. of moles of CO2
putting in the values leaves us with
Moles = 100/24000 = 1.67x10-3 moles of CO2
Now that we have the moles of both the compounds we can simply use the ratio method to find the moles of CO2 per unit CxHy i.e. X.
If 9.7x10-4 moles of P produce 1.67x10-3 moles of CO2, then 1 mole should produce 1.67x10-3/9.7x10-4 moles.
The answer you'll get will be 4.
part b)ii)
Now we have to find out how many moles of O2 have been used up.
As it's said, the O2 was provided in excess (200cm3).
But by the end the gas left was 50cm3.
So subtraction leaves us with 150cm3 of O2 used up.
We have the volume and the same molar law of volume applies to this gas as well
so we will be finding the no. of moles of O2 in the same way as CO2
the no. of moles will be 6