- Messages
- 603
- Reaction score
- 1,102
- Points
- 153
View attachment 62667 I need help with this. From March 2017 Paper 4 I only get 6 different carbon environments
We are currently struggling to cover the operational costs of Xtremepapers, as a result we might have to shut this website down. Please donate if we have helped you and help make a difference in other students' lives!
Click here to Donate Now (View Announcement)
View attachment 62667 I need help with this. From March 2017 Paper 4 I only get 6 different carbon environments

Isn't 6 and 7 the same?
Isn't 6 and 7 the same?
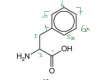
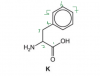
Thanks for the help, I understood it now.We should look beyond the immediate neighbors, 6 and 7 aren't exactly the same
The symmetry is at carbon 7, that is why 6 is the same as 6a and 5 is the same as 5a, but 7 is not the same as any of them
View attachment 62671
A simplified view of the molecule is shown before to hopefully explain the symmetry better, the symmetry is at carbon d.
View attachment 62672
I don't think you have to measure the exact spectra, your answer is right if you have taken your values from the Data Booklet.Someone please explain this to me. My answer was
-> Peak at 1670-1740 due to C=O
-> Peak at 3200-3600 due to RO-H
-> Peak at 2850-2950 due to C-H in alkane
I picked these range of wavenumber from Data booklet but mark scheme shows a different answer. For RO-H group, the absorption is 3200-3650 but data booklet shows 3200-3600... How do i measure the exact absorption from the spectra?
View attachment 62716
CAN ANYONE EXPLAIN HOW TO GET X AND Y?
but y?Anyone please help me with V and W ? Shouldn't Oxygen be replace with O-H bond ? View attachment 62717 View attachment 62718
I mean from C=O bond to C-OH bond or Am I wrong?but y?
but y?
For almost 10 years, the site XtremePapers has been trying very hard to serve its users.
However, we are now struggling to cover its operational costs due to unforeseen circumstances. If we helped you in any way, kindly contribute and be the part of this effort. No act of kindness, no matter how small, is ever wasted.
Click here to Donate Now