- Messages
- 29
- Reaction score
- 8
- Points
- 13
thnxx. cud u also tell y gly-ala is nt the same as ala-gly? (peptides)
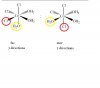
On left side is ammine and on right side is carboxylic in amino acid
Now ala-gla means ala's amine bonds with gla's acid group forming peptide
Gla-ala would be oppositte
Www.youtube.com/fahadsacademyonline
Www.fahadsacademy.com